当前位置:
X-MOL 学术
›
CrystEngComm
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structures and transistor properties of extended and unsymmetrical birhodanines
CrystEngComm ( IF 3.1 ) Pub Date : 2020-09-15 , DOI: 10.1039/d0ce01133a Yuji Sumimoto 1, 2, 3, 4 , Kodai Iijima 1, 2, 3, 4 , Dongho Yoo 1, 2, 3, 4 , Tadashi Kawamoto 1, 2, 3, 4 , Yann Le Gal 5, 6, 7, 8, 9 , Dominique Lorcy 5, 6, 7, 8, 9 , Takehiko Mori 1, 2, 3, 4
CrystEngComm ( IF 3.1 ) Pub Date : 2020-09-15 , DOI: 10.1039/d0ce01133a Yuji Sumimoto 1, 2, 3, 4 , Kodai Iijima 1, 2, 3, 4 , Dongho Yoo 1, 2, 3, 4 , Tadashi Kawamoto 1, 2, 3, 4 , Yann Le Gal 5, 6, 7, 8, 9 , Dominique Lorcy 5, 6, 7, 8, 9 , Takehiko Mori 1, 2, 3, 4
Affiliation
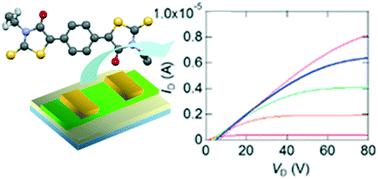
|
Birhodanines exhibit n-channel transistor properties with air stability. In this work, birhodanines with extended skeletons are investigated, in which a phenylene or quinoidal moiety is inserted into the central C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C part. We have also prepared N-phenyl and unsymmetrically N-substituted derivatives, including an unsubstituted N-H part. These compounds show n-channel transistor properties. In contrast to the herringbone structure of the parent compounds, the phenylene and phenyl compounds have stacking structures. The phenylene substitution decreases the acceptor ability, whereas the quinoidal substitution improves the acceptor ability and air stability of the transistors. The N-H derivative has a Z-form, suggesting the contribution of the enolic form, and the unsymmetrically N-substituted derivatives have double-layer structures with ordered alkyl chains.
C part. We have also prepared N-phenyl and unsymmetrically N-substituted derivatives, including an unsubstituted N-H part. These compounds show n-channel transistor properties. In contrast to the herringbone structure of the parent compounds, the phenylene and phenyl compounds have stacking structures. The phenylene substitution decreases the acceptor ability, whereas the quinoidal substitution improves the acceptor ability and air stability of the transistors. The N-H derivative has a Z-form, suggesting the contribution of the enolic form, and the unsymmetrically N-substituted derivatives have double-layer structures with ordered alkyl chains.
中文翻译:

扩展的和不对称的比罗丹宁的结构和晶体管特性
Birhodanines具有空气稳定性的n沟道晶体管特性。在这项工作中,研究了具有扩展骨架的二苯并丹宁,其中亚苯基或醌型部分插入到中央C![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) C部分。我们还制备了N-苯基和不对称的N-取代的衍生物,包括未取代的N- H部分。这些化合物显示出n沟道晶体管的特性。与母体化合物的人字形结构相反,亚苯基和苯基化合物具有堆积结构。亚苯基取代降低了受体的能力,而喹啉取代提高了晶体管的受体能力和空气稳定性。所述Ñ -H衍生物具有ž-α-形式,表明烯醇式的贡献,并且不对称的N-取代的衍生物具有带有有序的烷基链的双层结构。
C部分。我们还制备了N-苯基和不对称的N-取代的衍生物,包括未取代的N- H部分。这些化合物显示出n沟道晶体管的特性。与母体化合物的人字形结构相反,亚苯基和苯基化合物具有堆积结构。亚苯基取代降低了受体的能力,而喹啉取代提高了晶体管的受体能力和空气稳定性。所述Ñ -H衍生物具有ž-α-形式,表明烯醇式的贡献,并且不对称的N-取代的衍生物具有带有有序的烷基链的双层结构。
更新日期:2020-10-07
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C part. We have also prepared N-phenyl and unsymmetrically N-substituted derivatives, including an unsubstituted N-H part. These compounds show n-channel transistor properties. In contrast to the herringbone structure of the parent compounds, the phenylene and phenyl compounds have stacking structures. The phenylene substitution decreases the acceptor ability, whereas the quinoidal substitution improves the acceptor ability and air stability of the transistors. The N-H derivative has a Z-form, suggesting the contribution of the enolic form, and the unsymmetrically N-substituted derivatives have double-layer structures with ordered alkyl chains.
C part. We have also prepared N-phenyl and unsymmetrically N-substituted derivatives, including an unsubstituted N-H part. These compounds show n-channel transistor properties. In contrast to the herringbone structure of the parent compounds, the phenylene and phenyl compounds have stacking structures. The phenylene substitution decreases the acceptor ability, whereas the quinoidal substitution improves the acceptor ability and air stability of the transistors. The N-H derivative has a Z-form, suggesting the contribution of the enolic form, and the unsymmetrically N-substituted derivatives have double-layer structures with ordered alkyl chains.
中文翻译:

扩展的和不对称的比罗丹宁的结构和晶体管特性
Birhodanines具有空气稳定性的n沟道晶体管特性。在这项工作中,研究了具有扩展骨架的二苯并丹宁,其中亚苯基或醌型部分插入到中央C
![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) C部分。我们还制备了N-苯基和不对称的N-取代的衍生物,包括未取代的N- H部分。这些化合物显示出n沟道晶体管的特性。与母体化合物的人字形结构相反,亚苯基和苯基化合物具有堆积结构。亚苯基取代降低了受体的能力,而喹啉取代提高了晶体管的受体能力和空气稳定性。所述Ñ -H衍生物具有ž-α-形式,表明烯醇式的贡献,并且不对称的N-取代的衍生物具有带有有序的烷基链的双层结构。
C部分。我们还制备了N-苯基和不对称的N-取代的衍生物,包括未取代的N- H部分。这些化合物显示出n沟道晶体管的特性。与母体化合物的人字形结构相反,亚苯基和苯基化合物具有堆积结构。亚苯基取代降低了受体的能力,而喹啉取代提高了晶体管的受体能力和空气稳定性。所述Ñ -H衍生物具有ž-α-形式,表明烯醇式的贡献,并且不对称的N-取代的衍生物具有带有有序的烷基链的双层结构。



























 京公网安备 11010802027423号
京公网安备 11010802027423号