当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Glycoside hydrolase stabilization of transition state charge: new directions for inhibitor design
Chemical Science ( IF 8.4 ) Pub Date : 2020-09-16 , DOI: 10.1039/d0sc04401f Weiwu Ren 1, 2, 3, 4 , Marco Farren-Dai 1, 2, 3, 4 , Natalia Sannikova 1, 2, 3, 4 , Katarzyna Świderek 5, 6, 7, 8 , Yang Wang 1, 2, 3, 4 , Oluwafemi Akintola 1, 2, 3, 4 , Robert Britton 1, 2, 3, 4 , Vicent Moliner 5, 6, 7, 8 , Andrew J. Bennet 1, 2, 3, 4
Chemical Science ( IF 8.4 ) Pub Date : 2020-09-16 , DOI: 10.1039/d0sc04401f Weiwu Ren 1, 2, 3, 4 , Marco Farren-Dai 1, 2, 3, 4 , Natalia Sannikova 1, 2, 3, 4 , Katarzyna Świderek 5, 6, 7, 8 , Yang Wang 1, 2, 3, 4 , Oluwafemi Akintola 1, 2, 3, 4 , Robert Britton 1, 2, 3, 4 , Vicent Moliner 5, 6, 7, 8 , Andrew J. Bennet 1, 2, 3, 4
Affiliation
|
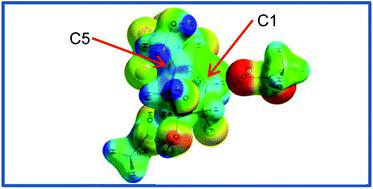
Carbasugars are structural mimics of naturally occurring carbohydrates that can interact with and inhibit enzymes involved in carbohydrate processing. In particular, carbasugars have attracted attention as inhibitors of glycoside hydrolases (GHs) and as therapeutic leads in several disease areas. However, it is unclear how the carbasugars are recognized and processed by GHs. Here, we report the synthesis of three carbasugar isotopologues and provide a detailed transition state (TS) analysis for the formation of the initial GH-carbasugar covalent intermediate, as well as for hydrolysis of this intermediate, using a combination of experimentally measured kinetic isotope effects and hybrid QM/MM calculations. We find that the α-galactosidase from Thermotoga maritima effectively stabilizes TS charge development on a remote C5-allylic center acting in concert with the reacting carbasugar, and catalysis proceeds via an exploded, or loose, SN2 transition state with no discrete enzyme-bound cationic intermediate. We conclude that, in complement to what we know about the TS structures of enzyme-natural substrate complexes, knowledge of the TS structures of enzymes reacting with non-natural carbasugar substrates shows that GHs can stabilize a wider range of positively charged TS structures than previously thought. Furthermore, this enhanced understanding will enable the design of new carbasugar GH transition state analogues to be used as, for example, chemical biology tools and pharmaceutical lead compounds.
中文翻译:

糖苷水解酶稳定过渡态电荷:抑制剂设计的新方向
糖食糖是天然存在的碳水化合物的结构模拟物,可以与碳水化合物相互作用并抑制涉及碳水化合物加工的酶。特别是,在许多疾病领域中,Carbonugars作为糖苷水解酶(GHs)的抑制剂和治疗先导引起了人们的关注。然而,目前尚不清楚GHs如何识别和处理碳糖。在这里,我们报告了三种羧甲基糖异向异构体的合成,并结合了实验测量的动力学同位素效应,为初始GH-碳糖共价中间体的形成以及该中间体的水解提供了详细的过渡态(TS)分析以及混合QM / MM计算。我们发现来自滨海嗜热菌的α-半乳糖苷酶有效地稳定了在远端C5烯丙基中心与反应的羧甲基纤维素相互作用的TS电荷的发展,并且催化反应通过爆炸或松散的S N 2过渡态进行,没有离散的与酶结合的阳离子中间体。我们得出结论,作为对酶-天然底物复合物TS结构的了解的补充,对与非天然Carbasugar底物反应的酶TS结构的了解表明,GHs可以稳定比以前更广泛的带正电的TS结构思想。此外,这种加深的理解将使新的卡巴苏加GH过渡态类似物的设计能够用作例如化学生物学工具和药物先导化合物。
更新日期:2020-10-07
中文翻译:

糖苷水解酶稳定过渡态电荷:抑制剂设计的新方向
糖食糖是天然存在的碳水化合物的结构模拟物,可以与碳水化合物相互作用并抑制涉及碳水化合物加工的酶。特别是,在许多疾病领域中,Carbonugars作为糖苷水解酶(GHs)的抑制剂和治疗先导引起了人们的关注。然而,目前尚不清楚GHs如何识别和处理碳糖。在这里,我们报告了三种羧甲基糖异向异构体的合成,并结合了实验测量的动力学同位素效应,为初始GH-碳糖共价中间体的形成以及该中间体的水解提供了详细的过渡态(TS)分析以及混合QM / MM计算。我们发现来自滨海嗜热菌的α-半乳糖苷酶有效地稳定了在远端C5烯丙基中心与反应的羧甲基纤维素相互作用的TS电荷的发展,并且催化反应通过爆炸或松散的S N 2过渡态进行,没有离散的与酶结合的阳离子中间体。我们得出结论,作为对酶-天然底物复合物TS结构的了解的补充,对与非天然Carbasugar底物反应的酶TS结构的了解表明,GHs可以稳定比以前更广泛的带正电的TS结构思想。此外,这种加深的理解将使新的卡巴苏加GH过渡态类似物的设计能够用作例如化学生物学工具和药物先导化合物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号