当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Glycan-Gold Nanoparticles as Multifunctional Probes for Multivalent Lectin-Carbohydrate Binding: Implications for Blocking Virus Infection and Nanoparticle Assembly
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-09-16 , DOI: 10.1021/jacs.0c06793 Darshita Budhadev 1 , Emma Poole 1 , Inga Nehlmeier 2 , Yuanyuan Liu 1 , James Hooper 3 , Elizabeth Kalverda 1 , Uchangi Satyaprasad Akshath 1 , Nicole Hondow 4 , W Bruce Turnbull 1 , Stefan Pöhlmann 2 , Yuan Guo 3 , Dejian Zhou 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-09-16 , DOI: 10.1021/jacs.0c06793 Darshita Budhadev 1 , Emma Poole 1 , Inga Nehlmeier 2 , Yuanyuan Liu 1 , James Hooper 3 , Elizabeth Kalverda 1 , Uchangi Satyaprasad Akshath 1 , Nicole Hondow 4 , W Bruce Turnbull 1 , Stefan Pöhlmann 2 , Yuan Guo 3 , Dejian Zhou 1
Affiliation
|
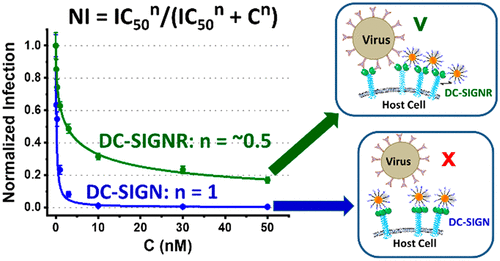
Multivalent lectin-glycan interactions are widespread in biology and are often exploited by pathogens to bind and infect host cells. Glycoconjugates can block such interactions and thereby prevent infection. The inhibition potency strongly de-pends on matching the spatial arrangement between the multivalent binding partners. However, the structural details of some key lectins remain unknown and different lectins may exhibit overlapping glycan specificity. This makes it difficult to design a glycoconjugate that can potently and specifically target a particular multimeric lectin for therapeutic interventions, especially under the challenging in vivo conditions. Conventional techniques such as surface plasmon resonance (SPR) and isothermal titration calorimetry (ITC) can provide quantitative binding thermodynamics and kinetics. However, they cannot reveal key structural information, e.g. lectin's binding site orientation, binding mode, and inter-binding site spacing, which are critical to design specific multivalent inhibitors. Herein we report that gold nanoparticles (GNPs) displaying a dense layer of simple glycans are powerful mechanistic probes for multivalent lectin-glycan interactions. They can not only quantify the GNP-glycan-lectin binding affinities via a new fluorescence quenching method, but also reveal drastically different affinity enhancing mechanisms between two closely-related tetrameric lectins, DC-SIGN (simultaneous binding to one GNP) and DC-SIGNR (inter-crosslinking with multiple GNPs), via a combined hydrodynamic size and electron microscopy analysis. Moreover, a new term, potential of assembly formation (PAF) has been proposed to successfully predict the assembly out-comes based on the binding mode between GNP-glycans and lectins. Finally, the GNP-glycans can potently and completely inhibit DC-SIGN-mediated augmentation of Ebola virus glycoprotein-driven cell entry (with IC50 values down to 95 pM), but only partially block DC-SIGNR-mediated virus infection. Our results suggest that the ability of a glycoconjugate to simulta-neously block all binding sites of a target lectin is key to robust inhibition of viral infection.
中文翻译:

聚糖-金纳米颗粒作为多价凝集素-碳水化合物结合的多功能探针:阻断病毒感染和纳米颗粒组装的意义
多价凝集素-聚糖相互作用在生物学中很普遍,并且经常被病原体利用来结合和感染宿主细胞。糖缀合物可以阻断这种相互作用,从而防止感染。抑制效力强烈依赖于匹配多价结合配偶体之间的空间排列。然而,一些关键凝集素的结构细节仍然未知,不同的凝集素可能表现出重叠的聚糖特异性。这使得很难设计出一种能够有效且特异性地靶向特定多聚体凝集素进行治疗干预的糖缀合物,尤其是在具有挑战性的体内条件下。诸如表面等离子体共振 (SPR) 和等温滴定量热法 (ITC) 等常规技术可以提供定量结合热力学和动力学。然而,它们不能揭示关键的结构信息,例如凝集素的结合位点方向、结合模式和结合位点间距,这些对于设计特定的多价抑制剂至关重要。在本文中,我们报告显示出致密的简单聚糖层的金纳米粒子 (GNP) 是用于多价凝集素-聚糖相互作用的强大机制探针。他们不仅可以通过一种新的荧光猝灭方法量化 GNP-聚糖-凝集素的结合亲和力,而且还揭示了两种密切相关的四聚体凝集素 DC-SIGN(同时结合到一个 GNP)和 DC-SIGNR 之间截然不同的亲和力增强机制(与多个 GNP 相互交联),通过结合流体动力学尺寸和电子显微镜分析。此外,还有一个新名词,组装形成潜力 (PAF) 已被提议基于 GNP-聚糖和凝集素之间的结合模式成功预测组装结果。最后,GNP-聚糖可以有效且完全地抑制 DC-SIGN 介导的埃博拉病毒糖蛋白驱动的细胞进入增强(IC50 值低至 95 pM),但只能部分阻断 DC-SIGNR 介导的病毒感染。我们的结果表明,糖缀合物同时阻断目标凝集素所有结合位点的能力是有效抑制病毒感染的关键。但只能部分阻断 DC-SIGNR 介导的病毒感染。我们的结果表明,糖缀合物同时阻断目标凝集素所有结合位点的能力是有效抑制病毒感染的关键。但只能部分阻断 DC-SIGNR 介导的病毒感染。我们的结果表明,糖缀合物同时阻断目标凝集素所有结合位点的能力是有效抑制病毒感染的关键。
更新日期:2020-09-16
中文翻译:

聚糖-金纳米颗粒作为多价凝集素-碳水化合物结合的多功能探针:阻断病毒感染和纳米颗粒组装的意义
多价凝集素-聚糖相互作用在生物学中很普遍,并且经常被病原体利用来结合和感染宿主细胞。糖缀合物可以阻断这种相互作用,从而防止感染。抑制效力强烈依赖于匹配多价结合配偶体之间的空间排列。然而,一些关键凝集素的结构细节仍然未知,不同的凝集素可能表现出重叠的聚糖特异性。这使得很难设计出一种能够有效且特异性地靶向特定多聚体凝集素进行治疗干预的糖缀合物,尤其是在具有挑战性的体内条件下。诸如表面等离子体共振 (SPR) 和等温滴定量热法 (ITC) 等常规技术可以提供定量结合热力学和动力学。然而,它们不能揭示关键的结构信息,例如凝集素的结合位点方向、结合模式和结合位点间距,这些对于设计特定的多价抑制剂至关重要。在本文中,我们报告显示出致密的简单聚糖层的金纳米粒子 (GNP) 是用于多价凝集素-聚糖相互作用的强大机制探针。他们不仅可以通过一种新的荧光猝灭方法量化 GNP-聚糖-凝集素的结合亲和力,而且还揭示了两种密切相关的四聚体凝集素 DC-SIGN(同时结合到一个 GNP)和 DC-SIGNR 之间截然不同的亲和力增强机制(与多个 GNP 相互交联),通过结合流体动力学尺寸和电子显微镜分析。此外,还有一个新名词,组装形成潜力 (PAF) 已被提议基于 GNP-聚糖和凝集素之间的结合模式成功预测组装结果。最后,GNP-聚糖可以有效且完全地抑制 DC-SIGN 介导的埃博拉病毒糖蛋白驱动的细胞进入增强(IC50 值低至 95 pM),但只能部分阻断 DC-SIGNR 介导的病毒感染。我们的结果表明,糖缀合物同时阻断目标凝集素所有结合位点的能力是有效抑制病毒感染的关键。但只能部分阻断 DC-SIGNR 介导的病毒感染。我们的结果表明,糖缀合物同时阻断目标凝集素所有结合位点的能力是有效抑制病毒感染的关键。但只能部分阻断 DC-SIGNR 介导的病毒感染。我们的结果表明,糖缀合物同时阻断目标凝集素所有结合位点的能力是有效抑制病毒感染的关键。


























 京公网安备 11010802027423号
京公网安备 11010802027423号