Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Controlling the Biological Fate of Micellar Nanoparticles: Balancing Stealth and Targeting.
ACS Nano ( IF 17.1 ) Pub Date : 2020-09-16 , DOI: 10.1021/acsnano.0c06033 Amal J Sivaram 1, 2, 3 , Andri Wardiana 1 , Sheilajen Alcantara 4 , Stefan E Sonderegger 1 , Nicholas L Fletcher 1, 2, 3 , Zachary H Houston 1, 2, 3 , Christopher B Howard 1, 5 , Stephen M Mahler 1, 5 , Cameron Alexander 6 , Stephen J Kent 4 , Craig A Bell 1, 2, 3 , Kristofer J Thurecht 1, 2, 3
ACS Nano ( IF 17.1 ) Pub Date : 2020-09-16 , DOI: 10.1021/acsnano.0c06033 Amal J Sivaram 1, 2, 3 , Andri Wardiana 1 , Sheilajen Alcantara 4 , Stefan E Sonderegger 1 , Nicholas L Fletcher 1, 2, 3 , Zachary H Houston 1, 2, 3 , Christopher B Howard 1, 5 , Stephen M Mahler 1, 5 , Cameron Alexander 6 , Stephen J Kent 4 , Craig A Bell 1, 2, 3 , Kristofer J Thurecht 1, 2, 3
Affiliation
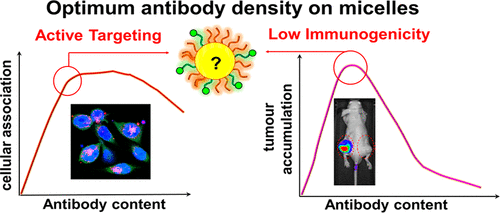
|
Integrating nanomaterials with biological entities has led to the development of diagnostic tools and biotechnology-derived therapeutic products. However, to optimize the design of these hybrid bionanomaterials, it is essential to understand how controlling the biological interactions will influence desired outcomes. Ultimately, this knowledge will allow more rapid translation from the bench to the clinic. In this paper, we developed a micellar system that was assembled using modular antibody–polymer amphiphilic materials. The amphiphilic nature was established using either poly(ethylene glycol) (PEG) or a single-chain variable fragment (scFv) from an antibody as the hydrophile and a thermoresponsive polymer (poly(oligoethylene glycol) methyl ether methacrylate) as the hydrophobe. By varying the ratios of these components, a series of nanoparticles with different antibody content was self-assembled, where the surface presentation of targeting ligand was carefully controlled. In vitro and in vivo analysis of these systems identified a mismatch between the optimal targeting ligand density to achieve maximum cell association in vitro compared to tumor accumulation in vivo. For this system, we determined an optimum antibody density for both longer circulation and enhanced targeting to tumors that balanced stealthiness of the particle (to evade immune recognition as determined in both mouse models and in whole human blood) with enhanced accumulation achieved through receptor binding on tumor cells in solid tumors. This approach provides fundamental insights into how different antibody densities affect the interaction of designed nanoparticles with both target cells and immune cells, thereby offering a method to probe the intricate interplay between increased targeting efficiency and the subsequent immune response to nanoparticles.
中文翻译:

控制胶束纳米粒子的生物命运:平衡隐身和目标。
纳米材料与生物实体的整合导致诊断工具和生物技术衍生的治疗产品的发展。但是,要优化这些杂化生物纳米材料的设计,必须了解控制生物相互作用将如何影响所需结果。最终,这些知识将使从工作台到诊所的翻译速度更快。在本文中,我们开发了使用模块化抗体-聚合物两亲性材料组装而成的胶束系统。使用聚(乙二醇)(PEG)或来自抗体的单链可变片段(scFv)作为亲水分子,并使用热响应性聚合物(聚(低聚乙二醇)甲基醚甲基丙烯酸酯)作为疏水基,可以建立两亲性质。通过改变这些成分的比例,这些系统的体外和体内分析发现,与体内肿瘤积累相比,最佳靶向配体密度在体外实现最大细胞缔合之间不匹配。对于该系统,我们确定了最佳的抗体密度,以延长循环并增强靶向肿瘤的能力,从而平衡颗粒的隐身性(以躲避小鼠模型和全人类血液中确定的免疫识别),并通过受体结合实现增强的积累实体瘤中的肿瘤细胞。这种方法为不同的抗体密度如何影响设计的纳米颗粒与靶细胞和免疫细胞之间的相互作用提供了基本的见识,从而提供了一种方法来探测增加的靶向效率与随后对纳米颗粒的免疫反应之间的复杂相互作用。
更新日期:2020-10-28
中文翻译:

控制胶束纳米粒子的生物命运:平衡隐身和目标。
纳米材料与生物实体的整合导致诊断工具和生物技术衍生的治疗产品的发展。但是,要优化这些杂化生物纳米材料的设计,必须了解控制生物相互作用将如何影响所需结果。最终,这些知识将使从工作台到诊所的翻译速度更快。在本文中,我们开发了使用模块化抗体-聚合物两亲性材料组装而成的胶束系统。使用聚(乙二醇)(PEG)或来自抗体的单链可变片段(scFv)作为亲水分子,并使用热响应性聚合物(聚(低聚乙二醇)甲基醚甲基丙烯酸酯)作为疏水基,可以建立两亲性质。通过改变这些成分的比例,这些系统的体外和体内分析发现,与体内肿瘤积累相比,最佳靶向配体密度在体外实现最大细胞缔合之间不匹配。对于该系统,我们确定了最佳的抗体密度,以延长循环并增强靶向肿瘤的能力,从而平衡颗粒的隐身性(以躲避小鼠模型和全人类血液中确定的免疫识别),并通过受体结合实现增强的积累实体瘤中的肿瘤细胞。这种方法为不同的抗体密度如何影响设计的纳米颗粒与靶细胞和免疫细胞之间的相互作用提供了基本的见识,从而提供了一种方法来探测增加的靶向效率与随后对纳米颗粒的免疫反应之间的复杂相互作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号