当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
NAD(P)H‐Dependent Enzymes for Reductive Amination: Active Site Description and Carbonyl‐Containing Compound Spectrum
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-09-16 , DOI: 10.1002/adsc.202000870 Laurine Ducrot 1 , Megan Bennett 2 , Gideon Grogan 2 , Carine Vergne‐Vaxelaire 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-09-16 , DOI: 10.1002/adsc.202000870 Laurine Ducrot 1 , Megan Bennett 2 , Gideon Grogan 2 , Carine Vergne‐Vaxelaire 1
Affiliation
|
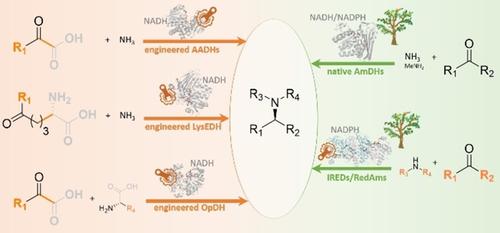
The biocatalytic asymmetric synthesis of amines from carbonyl compounds and amine precursors presents an important advance in sustainable synthetic chemistry. Oxidoreductases (ORs) that catalyze the NAD(P)H‐dependent reductive amination of carbonyl compounds directly to amines using amine donors present advantages complementary to those of amine transaminases (ATAs) with respect to selectivity, stability and substrate scope. Indeed some ORs accept alkyl and aryl amines as reaction partners enabling access to chiral secondary amine products that are not directly accessible using ATAs. Moreover, superior atom economy can usually be achieved as no sacrificial amines are required as with ATAs. In recent years a number of ORs that apparently catalyze both imine formation and imine reduction in the reductive amination of carbonyls has been identified using structure informed protein engineering, sequence analysis from natural biodiversity and increasingly a mixture of both. In this review we summarize the development of such enzymes from the engineering of amino acid dehydrogenases (AADHs) and opine dehydrogenases (OpDHs) to become amine dehydrogenases (AmDHs), which are active toward ketones devoid of any requisite carboxylate and/or amine functions, through to the discovery of native AmDHs and reductive aminases (RedAms), and the engineering of all of these scaffolds for improved or altered activity. Structural and mechanistic studies have revealed similarities, but also differences in the determinants of substrate binding and mechanism in the enzymes. The survey reveals that a complementary approach to enzyme discovery that utilizes both natural genetic resources and engineering can be combined to deliver biocatalysts that have significant potential for the industrial synthesis of chiral amines.
中文翻译:

NAD(P)H依赖的还原胺化酶:活性位点描述和含羰基的化合物光谱
由羰基化合物和胺前体的胺的生物催化不对称合成,在可持续合成化学中呈现出重要的进展。使用胺供体将羰基化合物的NAD(P)H依赖性胺催化直接还原为胺的氧化还原酶(OR)在选择性,稳定性和底物范围方面具有与胺转氨酶(ATA)互补的优势。实际上,一些OR接受烷基和芳基胺作为反应伙伴,从而能够获得使用ATA无法直接获得的手性仲胺产物。而且,由于不需要像ATA那样的牺牲胺,通常可以实现优异的原子经济性。近年来,已经使用结构知悉的蛋白质工程,天然生物多样性的序列分析以及越来越多的两者的混合物,鉴定出许多明显催化羰基还原胺化中亚胺形成和亚胺还原的OR。在这篇综述中,我们总结了从氨基酸脱氢酶(AADHs)和阿片脱氢酶(OpDHs)到胺脱氢酶(AmDHs)的工程化发展而来的此类酶,它们对没有任何必需的羧酸盐和/或胺功能的酮具有活性,直到发现天然的AmDHs和还原性氨化酶(RedAms),以及对所有这些支架进行工程改造以改善或改变活性。结构和力学研究发现相似之处,但是在底物结合的决定因素和酶的机制上也存在差异。调查显示,可以结合利用自然遗传资源和工程技术的补充酶发现方法,以提供对手性胺的工业合成具有重大潜力的生物催化剂。
更新日期:2020-09-16
中文翻译:

NAD(P)H依赖的还原胺化酶:活性位点描述和含羰基的化合物光谱
由羰基化合物和胺前体的胺的生物催化不对称合成,在可持续合成化学中呈现出重要的进展。使用胺供体将羰基化合物的NAD(P)H依赖性胺催化直接还原为胺的氧化还原酶(OR)在选择性,稳定性和底物范围方面具有与胺转氨酶(ATA)互补的优势。实际上,一些OR接受烷基和芳基胺作为反应伙伴,从而能够获得使用ATA无法直接获得的手性仲胺产物。而且,由于不需要像ATA那样的牺牲胺,通常可以实现优异的原子经济性。近年来,已经使用结构知悉的蛋白质工程,天然生物多样性的序列分析以及越来越多的两者的混合物,鉴定出许多明显催化羰基还原胺化中亚胺形成和亚胺还原的OR。在这篇综述中,我们总结了从氨基酸脱氢酶(AADHs)和阿片脱氢酶(OpDHs)到胺脱氢酶(AmDHs)的工程化发展而来的此类酶,它们对没有任何必需的羧酸盐和/或胺功能的酮具有活性,直到发现天然的AmDHs和还原性氨化酶(RedAms),以及对所有这些支架进行工程改造以改善或改变活性。结构和力学研究发现相似之处,但是在底物结合的决定因素和酶的机制上也存在差异。调查显示,可以结合利用自然遗传资源和工程技术的补充酶发现方法,以提供对手性胺的工业合成具有重大潜力的生物催化剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号