当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
One‐pot three‐component 1,3‐dipolar cycloaddition of azomethineylides to nitrofuran containing acetylenic ketones and molecular docking studies of the cycloadducts
Journal of Heterocyclic Chemistry ( IF 2.4 ) Pub Date : 2020-09-15 , DOI: 10.1002/jhet.4067 Anish Kumar Kadambar 1 , Balakrishna Kalluraya 1 , S. Madan Kumar 2
Journal of Heterocyclic Chemistry ( IF 2.4 ) Pub Date : 2020-09-15 , DOI: 10.1002/jhet.4067 Anish Kumar Kadambar 1 , Balakrishna Kalluraya 1 , S. Madan Kumar 2
Affiliation
|
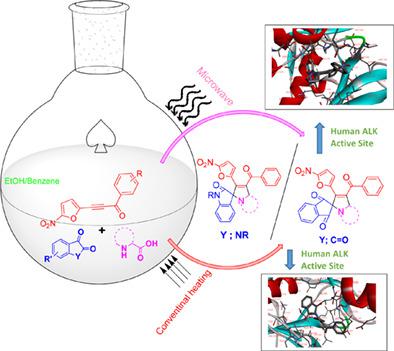
One‐pot three‐component 1,3‐dipolar cycloaddition reaction of azomethineylides generated from various combinations of isatin and ninhydrin with α‐aminoacids to 5‐nitrofurancontaining acetylenic ketones was carried out under thermal and microwave methods. The study of the regiochemical trend during the cycloaddition suggested that the nitrofuran ring exert no effect on the regiochemistry of the reaction as observed in the case of nitrofuran containing chalcones. The reason for the nil influence of the nitrofuran group is attributed to the increased electron density due to the triple bond. The newly synthesized compounds were docked to the active site of human anaplastic lymphoma kinase (ALK) to know the cancer cell toxicity in silico. The compounds 4b and 5a showed good binding interactions with the target in the active site.
中文翻译:

含甲炔酮的一锅三组分1,3-偶极偶氮甲亚胺与硝基呋喃的环加成反应和环加合物的分子对接研究
在热和微波方法下,将由靛红和茚三酮与α-氨基酸的各种组合生成的偶氮甲亚胺进行一锅三组分1,3-偶极环加成反应,生成含有5-硝基呋喃的炔酮。对环加成过程中区域化学趋势的研究表明,硝基呋喃环对反应的区域化学没有影响,就像在含硝基呋喃的查耳酮的情况下观察到的那样。硝基呋喃基团的影响为零的原因是由于三键导致电子密度增加。将新合成的化合物停靠在人类间变性淋巴瘤激酶(ALK)的活性位点上,以了解其对计算机的癌细胞毒性。化合物4b和5a 在活性位点与靶标表现出良好的结合相互作用。
更新日期:2020-11-12
中文翻译:

含甲炔酮的一锅三组分1,3-偶极偶氮甲亚胺与硝基呋喃的环加成反应和环加合物的分子对接研究
在热和微波方法下,将由靛红和茚三酮与α-氨基酸的各种组合生成的偶氮甲亚胺进行一锅三组分1,3-偶极环加成反应,生成含有5-硝基呋喃的炔酮。对环加成过程中区域化学趋势的研究表明,硝基呋喃环对反应的区域化学没有影响,就像在含硝基呋喃的查耳酮的情况下观察到的那样。硝基呋喃基团的影响为零的原因是由于三键导致电子密度增加。将新合成的化合物停靠在人类间变性淋巴瘤激酶(ALK)的活性位点上,以了解其对计算机的癌细胞毒性。化合物4b和5a 在活性位点与靶标表现出良好的结合相互作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号