当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and structure-activity relationship of 3-arylisoquinolone analogues as novel highly specific hCES2A inhibitors.
ChemMedChem ( IF 3.4 ) Pub Date : 2020-09-16 , DOI: 10.1002/cmdc.202000581 Yitian Zhao 1, 2 , Yuan Xiong 1 , Sanfeng Dong 1, 2 , Xiaoqing Guan 1 , Yunqing Song 1 , Yanqing Yang 2 , Kun Zou 2 , Zhao Li 2 , Yong Zhang 2 , Shengquan Fang 1 , Bo Li 2 , Weiliang Zhu 1, 2 , Kaixian Chen 1, 2 , Qi Jia 1 , Guangbo Ge 1
ChemMedChem ( IF 3.4 ) Pub Date : 2020-09-16 , DOI: 10.1002/cmdc.202000581 Yitian Zhao 1, 2 , Yuan Xiong 1 , Sanfeng Dong 1, 2 , Xiaoqing Guan 1 , Yunqing Song 1 , Yanqing Yang 2 , Kun Zou 2 , Zhao Li 2 , Yong Zhang 2 , Shengquan Fang 1 , Bo Li 2 , Weiliang Zhu 1, 2 , Kaixian Chen 1, 2 , Qi Jia 1 , Guangbo Ge 1
Affiliation
|
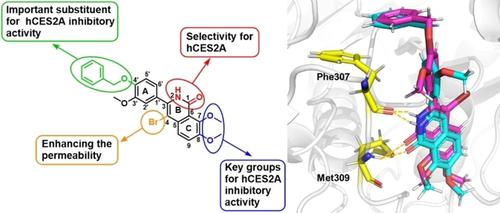
Mammalian carboxylesterases (CES) are key enzymes that participate in the hydrolytic metabolism of various endogenous and exogenous substrates. Human carboxylesterase 2A (hCES2A), mainly distributed in the small intestine and colon, plays a significant role in the hydrolysis of many drugs. In this study, 3‐arylisoquinolones 3 h [3‐(4‐(benzyloxy)‐3‐methoxyphenyl)‐7,8‐dimethoxyisoquinolin‐1(2H)‐one] and 4 a [3‐(4‐(benzyloxy)‐3‐methoxyphenyl)‐4‐bromo‐7,8‐dimethoxyisoquinolin‐1(2H)‐one] were found to have potent inhibitory effects on hCES2A (IC50=0.68 μΜ, Ki=0.36 μΜ) and excellent specificity (more than 147.05‐fold over hCES1 A). Moreover, 4 a exhibited threefold improved inhibition on intracellular hCES2A in living HepG2 cells relative to 3 h, with an IC50 value of 0.41 μΜ. Results of inhibition kinetics studies and molecular docking simulations demonstrate that both 3 h and 4 a can bind to multiple sites on hCES2A, functioning as mixed inhibitors. Structure−activity relationship analysis revealed that the lactam moiety on the B ring is crucial for specificity towards hCES2A, while a benzyloxy group is optimal for hCES2A inhibitory potency; the introduction of a bromine atom may enhance cell permeability, thereby increasing the intracellular hCES2A inhibitory activity.
中文翻译:

作为新型高度特异性 hCES2A 抑制剂的 3-芳基异喹诺酮类似物的合成和构效关系。
哺乳动物羧酸酯酶(CES)是参与各种内源性和外源性底物水解代谢的关键酶。人羧酸酯酶2A(hCES2A)主要分布在小肠和结肠中,在许多药物的水解中起重要作用。在这项研究中,3-芳基异喹诺酮3 h [3-(4-(苄氧基)-3-甲氧基苯基)-7,8-二甲氧基异喹啉-1(2 H )-one]和4 a [3-(4-(苄氧基) ‐3-甲氧基苯基)-4-溴-7,8-二甲氧基异喹啉-1(2 H )-one]被发现对 hCES2A 具有强效抑制作用 (IC 50 =0.68 μM, K i =0.36 μM) 和优异的特异性 (超过 hCES1 A 的 147.05 倍)。而且,图 4a显示出相对于3小时,对活HepG2细胞中的细胞内hCES2A的抑制提高了三倍,IC 50值为0.41μM。抑制动力学研究和分子对接模拟的结果表明,3 h和4 a都可以与 hCES2A 上的多个位点结合,起到混合抑制剂的作用。构效关系分析表明,B环上的内酰胺部分对hCES2A的特异性至关重要,而苄氧基对hCES2A抑制效力是最佳的;溴原子的引入可以增强细胞通透性,从而增加细胞内hCES2A的抑制活性。
更新日期:2020-09-16
中文翻译:

作为新型高度特异性 hCES2A 抑制剂的 3-芳基异喹诺酮类似物的合成和构效关系。
哺乳动物羧酸酯酶(CES)是参与各种内源性和外源性底物水解代谢的关键酶。人羧酸酯酶2A(hCES2A)主要分布在小肠和结肠中,在许多药物的水解中起重要作用。在这项研究中,3-芳基异喹诺酮3 h [3-(4-(苄氧基)-3-甲氧基苯基)-7,8-二甲氧基异喹啉-1(2 H )-one]和4 a [3-(4-(苄氧基) ‐3-甲氧基苯基)-4-溴-7,8-二甲氧基异喹啉-1(2 H )-one]被发现对 hCES2A 具有强效抑制作用 (IC 50 =0.68 μM, K i =0.36 μM) 和优异的特异性 (超过 hCES1 A 的 147.05 倍)。而且,图 4a显示出相对于3小时,对活HepG2细胞中的细胞内hCES2A的抑制提高了三倍,IC 50值为0.41μM。抑制动力学研究和分子对接模拟的结果表明,3 h和4 a都可以与 hCES2A 上的多个位点结合,起到混合抑制剂的作用。构效关系分析表明,B环上的内酰胺部分对hCES2A的特异性至关重要,而苄氧基对hCES2A抑制效力是最佳的;溴原子的引入可以增强细胞通透性,从而增加细胞内hCES2A的抑制活性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号