当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and Pharmacological Evaluation of σ2 Receptor Ligands Based on a 3‐Alkoxyisoxazole Scaffold: Potential Antitumor Effects against Osteosarcoma
ChemMedChem ( IF 3.4 ) Pub Date : 2020-09-15 , DOI: 10.1002/cmdc.202000461 Jun-Jie Shi 1 , Kun-Hang Jia 2 , Hao Sun 1 , Hendra Gunosewoyo 3 , Fan Yang 1 , Jie Tang 4 , Jian Luo 2 , Li-Fang Yu 1
ChemMedChem ( IF 3.4 ) Pub Date : 2020-09-15 , DOI: 10.1002/cmdc.202000461 Jun-Jie Shi 1 , Kun-Hang Jia 2 , Hao Sun 1 , Hendra Gunosewoyo 3 , Fan Yang 1 , Jie Tang 4 , Jian Luo 2 , Li-Fang Yu 1
Affiliation
|
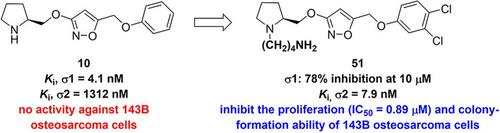
Since its initial discovery as the basis for nicotinic acetylcholine receptor ligands, the 3‐alkoxyisoxazole scaffold has been shown to be a versatile platform for the development of potent σ1 and σ2 receptor ligands. Herein we report a further SAR exploration of the 3‐alkoxyisoxazole scaffold with the aim of obtaining potent σ2 receptor ligands. Various substitutions on the benzene ring and at the basic amino regions resulted in a total of 21 compounds that were tested for their binding affinities for the σ2 receptor. In particular, compound 51 [(2S)‐1‐(4‐ammoniobutyl)‐2‐(((5‐((3,4‐dichlorophenoxy)methyl)isoxazol‐3‐yl)oxy)methyl)pyrrolidin‐1‐ium chloride] was identified as one of the most potent σ2 ligands within the series, with a Ki value of 7.9 nM. It demonstrated potent antiproliferative effects on both osteosarcoma cell lines 143B and MOS−J (IC50 values of 0.89 and 0.71 μM, respectively), relative to siramesine (IC50 values of 1.81 and 2.01 μM). Moreover, compound 51 inhibited clonal formation of osteosarcoma 143B cells at 1 μM, corresponding to half the dose required of siramesine for similar effects. The general cytotoxicity profile of compound 51 was assessed in a number of normal cell lines, including HaCaT, HAF, and LO2 cells. Furthermore, FACS analysis showed that compound 51 likely inhibits osteosarcoma cell growth by disruption of the cell cycle and promotion of apoptosis.
中文翻译:

基于 3-烷氧基异恶唑支架的 σ2 受体配体的合成和药理学评价:对骨肉瘤的潜在抗肿瘤作用
自从最初发现作为烟碱型乙酰胆碱受体配体的基础以来,3-烷氧基异恶唑支架已被证明是开发有效的 σ1 和 σ2 受体配体的通用平台。在此,我们报告了对 3-烷氧基异恶唑支架的进一步 SAR 探索,目的是获得有效的 σ2 受体配体。苯环上和碱性氨基区域上的各种取代产生了总共 21 种化合物,用于测试它们对 σ2 受体的结合亲和力。特别是化合物51 [(2 S )-1-(4-氨丁基)-2-(((5-((3,4-dichlorophenoxy)methyl)isoxazol-3-yl)oxy)methyl)pyrrolidin-1-氯化鎓] 被确定为该系列中最有效的 σ2 配体之一,其K i值 7.9 nM。相对于西拉美新(IC 50值分别为 1.81 和 2.01 μM),它对骨肉瘤细胞系 143B 和 MOS-J(IC 50值分别为 0.89 和 0.71 μM)显示出有效的抗增殖作用。此外,化合物51以 1 μM 抑制骨肉瘤 143B 细胞的克隆形成,相当于西拉美新达到类似效果所需剂量的一半。在许多正常细胞系中评估了化合物51的一般细胞毒性特征,包括 HaCaT、HAF 和 LO2 细胞。此外,FACS 分析表明,化合物51可能通过破坏细胞周期和促进细胞凋亡来抑制骨肉瘤细胞生长。
更新日期:2020-09-15
中文翻译:

基于 3-烷氧基异恶唑支架的 σ2 受体配体的合成和药理学评价:对骨肉瘤的潜在抗肿瘤作用
自从最初发现作为烟碱型乙酰胆碱受体配体的基础以来,3-烷氧基异恶唑支架已被证明是开发有效的 σ1 和 σ2 受体配体的通用平台。在此,我们报告了对 3-烷氧基异恶唑支架的进一步 SAR 探索,目的是获得有效的 σ2 受体配体。苯环上和碱性氨基区域上的各种取代产生了总共 21 种化合物,用于测试它们对 σ2 受体的结合亲和力。特别是化合物51 [(2 S )-1-(4-氨丁基)-2-(((5-((3,4-dichlorophenoxy)methyl)isoxazol-3-yl)oxy)methyl)pyrrolidin-1-氯化鎓] 被确定为该系列中最有效的 σ2 配体之一,其K i值 7.9 nM。相对于西拉美新(IC 50值分别为 1.81 和 2.01 μM),它对骨肉瘤细胞系 143B 和 MOS-J(IC 50值分别为 0.89 和 0.71 μM)显示出有效的抗增殖作用。此外,化合物51以 1 μM 抑制骨肉瘤 143B 细胞的克隆形成,相当于西拉美新达到类似效果所需剂量的一半。在许多正常细胞系中评估了化合物51的一般细胞毒性特征,包括 HaCaT、HAF 和 LO2 细胞。此外,FACS 分析表明,化合物51可能通过破坏细胞周期和促进细胞凋亡来抑制骨肉瘤细胞生长。


























 京公网安备 11010802027423号
京公网安备 11010802027423号