当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of Extended Bipyridine‐proline Chiral Catalysts and Resulting Effects on the Asymmetric Aldol Reactions of Bulkier Aldehyde Derivatives with Cyclohexanone
ChemistrySelect ( IF 2.1 ) Pub Date : 2020-09-16 , DOI: 10.1002/slct.202002956 Guangpeng Xu 1 , Yajing Zhang 1 , Jihong Sun 1 , Shiyang Bai 1 , Hongwu Zhao 1
ChemistrySelect ( IF 2.1 ) Pub Date : 2020-09-16 , DOI: 10.1002/slct.202002956 Guangpeng Xu 1 , Yajing Zhang 1 , Jihong Sun 1 , Shiyang Bai 1 , Hongwu Zhao 1
Affiliation
|
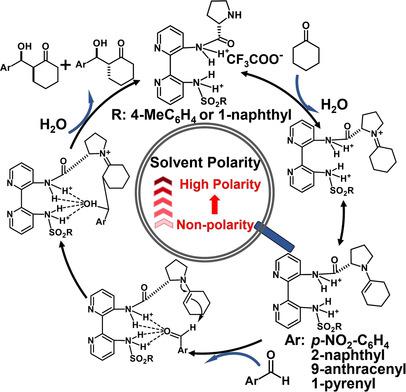
Axially‐unfixed 2,2′‐bipyridine‐based chiral catalysts were synthesized using enantiopure amino acids as chiral sources, which were successfully used in asymmetric aldol reactions of p‐nitrobenzaldehyde with cyclohexanone, while the bulkier aldehyde derivatives (2‐naphthaldehyde, 9‐anthracenecarboxaldehyde, and 1‐pyrenecarboxaldehyde) were selected to further elucidate the catalytic properties. Particularly, the influences of the bipyridine‐proline chiral structures and the polarities of used solvents (petroleum ether, toluene, CH2Cl2, ethanol, DMF, DMSO, and water) on the catalytic performance were investigated. The results indicated that the structure of bipyridine catalysts, the molecular volume of aldehydes, and the polarity of solvents have significant effects on the catalytic activities, in which, the smaller steric effects were conducive to the improvement of the yields and stereoselectivities for asymmetric aldol reaction, along with the increased polarity of used solvents and the decreased molecular volume of aldehydes. Meanwhile, the chemical identity of all compounds was confirmed by 1H‐NMR, 13C‐NMR, HRMS, and HPLC analysis.
中文翻译:

扩展联吡啶-脯氨酸手性催化剂的合成及其对较大醛衍生物与环己酮的不对称羟醛反应的影响
以对映纯氨基酸为手性来源合成了轴向未固定的2,2'-联吡啶手性催化剂,该催化剂成功用于对硝基苯甲醛与环己酮的不对称羟醛反应,而较大的醛衍生物(2-萘醛,9-选择蒽蒽甲醛和1-吡啶甲醛)以进一步阐明催化性能。特别是联吡啶-脯氨酸手性结构和所用溶剂(石油醚,甲苯,CH 2 Cl 2,乙醇,DMF,DMSO和水)对催化性能的影响。结果表明,联吡啶催化剂的结构,醛的分子体积和溶剂的极性对催化活性有重要影响,其中较小的空间效应有利于提高不对称醛醇缩合反应的收率和立体选择性。 ,以及所用溶剂的极性增加和醛的分子体积减少。同时,通过1 H-NMR,13 C-NMR,HRMS和HPLC分析确认了所有化合物的化学同一性。
更新日期:2020-09-16
中文翻译:

扩展联吡啶-脯氨酸手性催化剂的合成及其对较大醛衍生物与环己酮的不对称羟醛反应的影响
以对映纯氨基酸为手性来源合成了轴向未固定的2,2'-联吡啶手性催化剂,该催化剂成功用于对硝基苯甲醛与环己酮的不对称羟醛反应,而较大的醛衍生物(2-萘醛,9-选择蒽蒽甲醛和1-吡啶甲醛)以进一步阐明催化性能。特别是联吡啶-脯氨酸手性结构和所用溶剂(石油醚,甲苯,CH 2 Cl 2,乙醇,DMF,DMSO和水)对催化性能的影响。结果表明,联吡啶催化剂的结构,醛的分子体积和溶剂的极性对催化活性有重要影响,其中较小的空间效应有利于提高不对称醛醇缩合反应的收率和立体选择性。 ,以及所用溶剂的极性增加和醛的分子体积减少。同时,通过1 H-NMR,13 C-NMR,HRMS和HPLC分析确认了所有化合物的化学同一性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号