当前位置:
X-MOL 学术
›
Adv. Healthcare Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enabling MedTech Translation in Academia: Redefining Value Proposition with Updated Regulations.
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2020-09-16 , DOI: 10.1002/adhm.202001237 Didier Letourneur 1 , Kieran Joyce 2, 3 , Cédric Chauvierre 1 , Yves Bayon 4 , Abhay Pandit 2
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2020-09-16 , DOI: 10.1002/adhm.202001237 Didier Letourneur 1 , Kieran Joyce 2, 3 , Cédric Chauvierre 1 , Yves Bayon 4 , Abhay Pandit 2
Affiliation
|
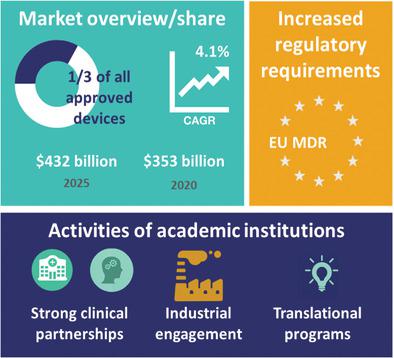
Academic institutions are becoming more focused on translating new technologies for clinical applications. A transition from “bench to bedside” is often described to take basic research concepts and methods to develop a therapeutic or diagnostic solution with proven evidence of efficacy at the clinical level while also fulfilling regulatory requirements. The regulatory environment is evolving in Europe with transition and grace periods for the full enforcement of the Medical Device Regulation 2017/745 (MDR), replacing the Medical Device Directive 93/42/EEC (MDD). These new guidelines increase demands for scientific, technical, and clinical data with reduced capacity in regulatory bodies creating uncertainty in future product certification. Academic translational activities will be uniquely affected by this new legislation. The barriers and threats to successful translation in academia can be overcome by strong clinical partnerships, close‐industrial collaborations, and entrepreneurial programs, enabling continued product development to overcome regulatory hurdles, reassuring their foothold of medical device development.
中文翻译:

在学术界实现MedTech翻译:通过更新的法规重新定义价值主张。
学术机构越来越专注于为临床应用翻译新技术。通常描述从“长凳到床头”的过渡采用基本的研究概念和方法来开发治疗或诊断解决方案,并在临床水平上证明其功效,同时还满足监管要求。在欧洲,监管环境正在发生变化,过渡期和宽限期为全面执行《 2017/745医疗器械法规》(取代《医疗器械指令》 93/42 / EEC(MDD))。这些新准则增加了对科学,技术和临床数据的需求,而监管机构的能力却下降了,从而给未来的产品认证带来了不确定性。这一新法规将对学术翻译活动产生独特的影响。
更新日期:2020-09-16
中文翻译:

在学术界实现MedTech翻译:通过更新的法规重新定义价值主张。
学术机构越来越专注于为临床应用翻译新技术。通常描述从“长凳到床头”的过渡采用基本的研究概念和方法来开发治疗或诊断解决方案,并在临床水平上证明其功效,同时还满足监管要求。在欧洲,监管环境正在发生变化,过渡期和宽限期为全面执行《 2017/745医疗器械法规》(取代《医疗器械指令》 93/42 / EEC(MDD))。这些新准则增加了对科学,技术和临床数据的需求,而监管机构的能力却下降了,从而给未来的产品认证带来了不确定性。这一新法规将对学术翻译活动产生独特的影响。


























 京公网安备 11010802027423号
京公网安备 11010802027423号