Journal of Colloid and Interface Science ( IF 9.9 ) Pub Date : 2020-09-16 , DOI: 10.1016/j.jcis.2020.09.022 Hongmin Cui , Jianguo Xu , Jinsong Shi , Shengyong You , Chao Zhang , Nanfu Yan , Yuewei Liu , Guihua Chen
|
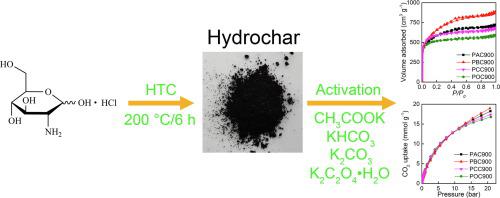
KOH is one of the most widely used activators for the synthesis of highly porous carbon. However, the strong causticity of KOH could cause serious equipment damage and safety issues at high temperature. In the current work, we presented the synthesis of porous carbons with large surface area using four different potassium salts (CH3COOK, KHCO3, K2CO3, and K2C2O4·H2O) as mild but effective activators. Hydrochar prepared from the hydrothermal carbonization of glucosamine hydrochloride was used as carbon precursor. The carbons exhibited specific surface area up to 2403 m2/g. In order to reveal the different influences of nitrogen doping and textural properties under low and high pressure conditions, CO2 adsorption was tested with pressure up to 20 bar. At 1 bar, ultramicropore was the most determinant factor. Nitrogen doping also showed important influences, especially on the CO2/N2 selectivity. At 20 bar, the carbon activated by KHCO3 showed CO2 uptakes of 26.24 (0 °C) and 18.63 mmol/g (25 °C). The experiment results indicated that the uptake at 20 bar correlated with the total surface area and total porosity of the carbon, and no apparent effects from nitrogen doping were observed.
中文翻译:

不同钾盐作为多孔碳活化剂的评价及其在CO 2吸附中的应用
KOH是用于合成高度多孔碳的最广泛使用的活化剂之一。但是,KOH的强腐蚀性可能会导致严重的设备损坏以及高温下的安全问题。在当前的工作中,我们提出了使用四种不同的钾盐(CH 3 COOK,KHCO 3,K 2 CO 3和K 2 C 2 O 4 ·H 2 O)温和但有效的大表面积多孔碳合成方法。激活剂。将由氨基葡萄糖盐酸盐的水热碳化制备的烃用作碳前体。碳的比表面积高达2403 m 2/G。为了揭示在低压和高压条件下氮掺杂和质构特性的不同影响,在最高20 bar的压力下测试了CO 2吸附。在1 bar时,超微孔是最决定因素。氮掺杂也显示出重要的影响,特别是对CO 2 / N 2的选择性。在20 bar下,被KHCO 3活化的碳显示出26.24(0°C)和18.63 mmol / g(25°C)的CO 2吸收。实验结果表明,在20 bar下的吸收与碳的总表面积和总孔隙率相关,并且未观察到氮掺杂的明显影响。



























 京公网安备 11010802027423号
京公网安备 11010802027423号