当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Time-Dependent Lipid Dynamics, Organization and Peptide-Lipid Interaction in Phospholipid Bilayers with Incorporated β-Amyloid Oligomers.
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2020-09-15 , DOI: 10.1021/acs.jpclett.0c01967 Wei Qiang 1 , Katelynne E Doherty 1 , Lukas M Klees 1 , Yuto Tobin-Miyaji 1
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2020-09-15 , DOI: 10.1021/acs.jpclett.0c01967 Wei Qiang 1 , Katelynne E Doherty 1 , Lukas M Klees 1 , Yuto Tobin-Miyaji 1
Affiliation
|
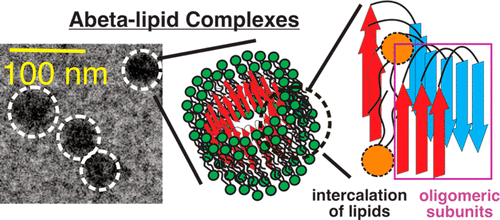
Nonfibrillar β-amyloid (Aβ) oligomers are considered as major neurotoxic species in the pathology of Alzheimer’s disease. The presence of Aβ oligomers was shown to cause membrane disruptions in a broad range of model systems. However, the molecular basis of such a disruption process remains unknown. We previously demonstrated that membrane-incorporated 40-residue Aβ (Aβ40) oligomers could form coaggregates with phospholipids. This process occurred more rapidly than the fibrillization of Aβ40 and led to more severe membrane disruption. The present study probes the time-dependent changes in lipid dynamics, bilayer structures, and peptide-lipid interactions along the time course of the oligomer-induced membrane disruption, using solid-state NMR spectroscopy. Our results suggest the presence of certain intermediate states with phospholipid molecules entering the C-terminal hydrogen-bonding networks of the Aβ40 oligomeric cores. This work provides insights on the molecular mechanisms of Aβ40-oligomer-induced membrane disruption.
中文翻译:

与掺入β-淀粉样蛋白寡聚体的磷脂双层膜中随时间变化的脂质动力学,组织和肽-脂质相互作用。
非原纤维β-淀粉样蛋白(Aβ)低聚物被认为是阿尔茨海默氏病病理学中的主要神经毒性物质。在广泛的模型系统中,显示Aβ低聚物的存在会引起膜破裂。然而,这种破坏过程的分子基础仍然未知。我们先前证明,膜结合的40残基Aβ(Aβ40)低聚物可与磷脂形成共聚集体。这个过程比Aβ40的原纤维化更快并导致更严重的膜破裂。本研究使用固态NMR光谱探测了寡聚物诱导的膜破裂的时间过程中脂质动力学,双层结构和肽-脂相互作用的时间依赖性变化。我们的结果表明存在某些中间状态,其中磷脂分子进入Aβ40低聚核心的C端氢键网络。这项工作提供了对Aβ的分子机制的见解40 -oligomer诱导膜破坏。
更新日期:2020-10-02
中文翻译:

与掺入β-淀粉样蛋白寡聚体的磷脂双层膜中随时间变化的脂质动力学,组织和肽-脂质相互作用。
非原纤维β-淀粉样蛋白(Aβ)低聚物被认为是阿尔茨海默氏病病理学中的主要神经毒性物质。在广泛的模型系统中,显示Aβ低聚物的存在会引起膜破裂。然而,这种破坏过程的分子基础仍然未知。我们先前证明,膜结合的40残基Aβ(Aβ40)低聚物可与磷脂形成共聚集体。这个过程比Aβ40的原纤维化更快并导致更严重的膜破裂。本研究使用固态NMR光谱探测了寡聚物诱导的膜破裂的时间过程中脂质动力学,双层结构和肽-脂相互作用的时间依赖性变化。我们的结果表明存在某些中间状态,其中磷脂分子进入Aβ40低聚核心的C端氢键网络。这项工作提供了对Aβ的分子机制的见解40 -oligomer诱导膜破坏。


























 京公网安备 11010802027423号
京公网安备 11010802027423号