当前位置:
X-MOL 学术
›
J. Alloys Compd.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Improved electrochemical performance of LiMn1.5M0.5O4 (M=Ni, Co, Cu) based cathodes for lithium-ion batteries
Journal of Alloys and Compounds ( IF 6.2 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.jallcom.2020.157208 Renato Gonçalves , Poonam Sharma , Pura Ram , Stanislav Ferdov , M. Manuela Silva , Carlos M. Costa , Rahul Singhal , Rakesh K. Sharma , Senentxu Lanceros-Méndez
Journal of Alloys and Compounds ( IF 6.2 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.jallcom.2020.157208 Renato Gonçalves , Poonam Sharma , Pura Ram , Stanislav Ferdov , M. Manuela Silva , Carlos M. Costa , Rahul Singhal , Rakesh K. Sharma , Senentxu Lanceros-Méndez
|
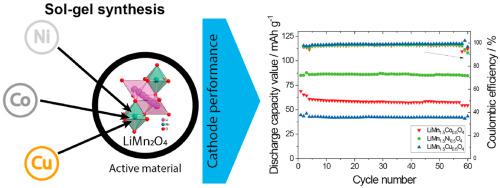
Abstract LiMn2O4 and LiMn1.5M0.5O4 (M: Ni, Cu, Co) doped particles have been synthetized by sol-gel. Particles between 50 and 200 nm were obtained with the cubic spinel structure of the LiMn2O4. Ni doping shows a more efficient substitution in the octahedral 16d site, replacing the Mn3+ ion, improving the important drawback of poor cycling behavior of LiMn2O4. The average pore size decrease with the addition of the doped elements in the LiMn2O4 structure from 2.9 to 2.6 nm. Thermal analysis shows that the doped particles present higher thermal stability that the undoped ones. Electrochemical behavior of the cathodes prepared with each of the active materials show that the doping influenced the electrochemical performance of the active material. Thus, a specific capacity of 33, 74, 44 and 53 mAh g−1 (at C) and 74, 89, 59 and 69 mAh g−1 (at C/10) were obtained for LiMn2O4, LiMn1.5Ni0.5O4, LiMn1.5Cu0.5O4 and LiMn1.5Co0.5O4 cathodes, respectively. All cathodes present good electrochemical stability with low capacity fade of 0.5 and 3.1% for LiMn1.5Ni0.5O4 and LiMn1.5Cu0.5O4, respectively, after 50 cycles. These results show an improvement of electrochemical performance for LiMn2O4 doped with Ni, Cu and Co, demonstrating their suitability for lithium-ion battery systems.
中文翻译:

用于锂离子电池的 LiMn1.5M0.5O4 (M=Ni, Co, Cu) 基正极的电化学性能改进
摘要 采用溶胶-凝胶法合成了LiMn2O4和LiMn1.5M0.5O4(M:Ni,Cu,Co)掺杂粒子。获得具有 LiMn2O4 立方尖晶石结构的 50 和 200 nm 之间的颗粒。Ni 掺杂在八面体 16d 位点显示出更有效的取代,取代了 Mn3+ 离子,改善了 LiMn2O4 循环性能差的重要缺点。随着 LiMn2O4 结构中掺杂元素的加入,平均孔径从 2.9 到 2.6 nm。热分析表明,掺杂颗粒比未掺杂颗粒表现出更高的热稳定性。用每种活性材料制备的正极的电化学行为表明,掺杂影响了活性材料的电化学性能。因此,比容量为 33、74、44 和 53 mAh g-1(在 C 下)和 74、89,对于 LiMn2O4、LiMn1.5Ni0.5O4、LiMn1.5Cu0.5O4 和 LiMn1.5Co0.5O4 阴极,分别获得了 59 和 69 mAh g-1(在 C/10 下)。所有正极均表现出良好的电化学稳定性,LiMn1.5Ni0.5O4 和 LiMn1.5Cu0.5O4 在 50 次循环后分别具有 0.5% 和 3.1% 的低容量衰减。这些结果表明,掺杂有 Ni、Cu 和 Co 的 LiMn2O4 的电化学性能有所改善,证明它们适用于锂离子电池系统。
更新日期:2021-02-01
中文翻译:

用于锂离子电池的 LiMn1.5M0.5O4 (M=Ni, Co, Cu) 基正极的电化学性能改进
摘要 采用溶胶-凝胶法合成了LiMn2O4和LiMn1.5M0.5O4(M:Ni,Cu,Co)掺杂粒子。获得具有 LiMn2O4 立方尖晶石结构的 50 和 200 nm 之间的颗粒。Ni 掺杂在八面体 16d 位点显示出更有效的取代,取代了 Mn3+ 离子,改善了 LiMn2O4 循环性能差的重要缺点。随着 LiMn2O4 结构中掺杂元素的加入,平均孔径从 2.9 到 2.6 nm。热分析表明,掺杂颗粒比未掺杂颗粒表现出更高的热稳定性。用每种活性材料制备的正极的电化学行为表明,掺杂影响了活性材料的电化学性能。因此,比容量为 33、74、44 和 53 mAh g-1(在 C 下)和 74、89,对于 LiMn2O4、LiMn1.5Ni0.5O4、LiMn1.5Cu0.5O4 和 LiMn1.5Co0.5O4 阴极,分别获得了 59 和 69 mAh g-1(在 C/10 下)。所有正极均表现出良好的电化学稳定性,LiMn1.5Ni0.5O4 和 LiMn1.5Cu0.5O4 在 50 次循环后分别具有 0.5% 和 3.1% 的低容量衰减。这些结果表明,掺杂有 Ni、Cu 和 Co 的 LiMn2O4 的电化学性能有所改善,证明它们适用于锂离子电池系统。

























 京公网安备 11010802027423号
京公网安备 11010802027423号