Cell Reports ( IF 8.8 ) Pub Date : 2020-09-15 , DOI: 10.1016/j.celrep.2020.108154 Kim Schneider 1 , Geoffrey Michael Nelson 1 , Joseph Luke Watson 1 , Jörg Morf 2 , Maximillian Dalglish 1 , Laura Martina Luh 1 , Annika Weber 1 , Anne Bertolotti 1
|
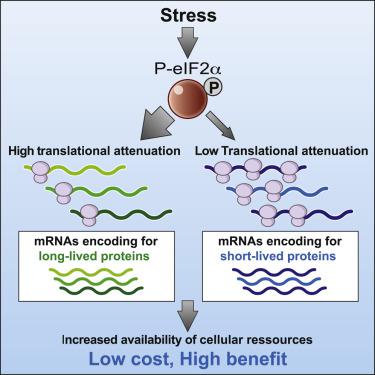
Phosphorylation of the translation initiation factor eIF2α is a rapid and vital response to many forms of stress, including protein-misfolding stress in the endoplasmic reticulum (ER stress). It is believed to cause a general reduction in protein synthesis while enabling translation of few transcripts. Such a reduction of protein synthesis comes with the threat of depleting essential proteins, a risk thought to be mitigated by its transient nature. Here, we find that translation attenuation is not uniform, with cytosolic and mitochondrial ribosomal subunits being prominently downregulated. Translation attenuation of these targets persists after translation recovery. Surprisingly, this occurs without a measurable decrease in ribosomal proteins. Explaining this conundrum, translation attenuation preferentially targets long-lived proteins, a finding not only demonstrated by ribosomal proteins but also observed at a global level. This shows that protein stability buffers the cost of translational attenuation, establishing an evolutionary principle of cellular robustness.
中文翻译:

蛋白质稳定性可缓冲eIF2α磷酸化后翻译减弱的成本。
翻译起始因子eIF2α的磷酸化是对多种形式压力的快速而重要的响应,包括内质网中的蛋白质错误折叠应力(ER应力)。据信,这导致蛋白质合成的普遍减少,同时能够翻译很少的转录本。蛋白质合成的这种减少伴随着消耗必需蛋白质的威胁,这种风险被其暂时性所减轻。在这里,我们发现翻译衰减不均匀,胞质和线粒体核糖体亚基被显着下调。这些目标的翻译衰减在翻译恢复后仍然存在。出乎意料的是,这种情况在核糖体蛋白没有明显减少的情况下发生。解释这一难题,翻译衰减优先针对长寿蛋白,这一发现不仅通过核糖体蛋白得到证实,而且还在全球范围内得到了观察。这表明蛋白质稳定性缓冲了翻译减弱的成本,建立了细胞健壮性的进化原理。



























 京公网安备 11010802027423号
京公网安备 11010802027423号