当前位置:
X-MOL 学术
›
Sustain. Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Elucidating hydrogen storage properties of two-dimensional siligraphene (SiC8) monolayers upon selected metal decoration
Sustainable Energy & Fuels ( IF 5.6 ) Pub Date : 2020-09-14 , DOI: 10.1039/d0se00852d Ekaterina Anikina 1, 2, 3, 4, 5 , Tanveer Hussain 6, 7, 8, 9 , Valery Beskachko 1, 2, 3, 4 , Rajeev Ahuja 5, 10, 11, 12, 13
Sustainable Energy & Fuels ( IF 5.6 ) Pub Date : 2020-09-14 , DOI: 10.1039/d0se00852d Ekaterina Anikina 1, 2, 3, 4, 5 , Tanveer Hussain 6, 7, 8, 9 , Valery Beskachko 1, 2, 3, 4 , Rajeev Ahuja 5, 10, 11, 12, 13
Affiliation
|
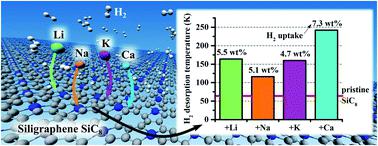
Density functional theory calculations with dispersion corrections were employed to investigate the hydrogen (H2) adsorptive properties of siligraphene (SiC8), pristine and decorated with selected alkali (Li, Na, and K) and alkaline-earth (Be, Mg, and Ca) metals. We found that all the considered metals (Me), except Mg and Be, bind strongly to SiC8 even at high doping concentrations (SiC8Me2) by donating a major portion of their valence electrons to SiC8. Ab initio molecular dynamics (AIMD) simulations confirmed the thermal stabilities of SiC8Me2 (Me = Li, Na, K, Ca) at 300 K. We showed that Li, Na, and Ca-doped SiC8 adsorbed multiple H2 molecules with binding energies (Ebind) at least two times stronger than that of the pristine SiC8 (Epristinebind = −70 meV per H2). Overall, both SiC8Li2 and SiC8Ca2 adsorbed two and four H2 molecules per metal adatom, respectively, having Ebind within the desirable range for an effective adsorption/desorption process. The resulting gravimetric densities of SiC8Li2 and SiC8Ca2 were 5.5 wt% and 7.3 wt%, respectively, surpassing the U.S. Department of Energy's 2025 goal of 5.5 wt%. The estimated H2 desorption temperatures exceed substantially the boiling point of liquid nitrogen, confirming the potential of light metal decorated SiC8 as a promising material for H2 storage.
中文翻译:

在选择金属装饰后阐明二维硅单晶(SiC8)单层的储氢特性
与色散校正密度泛函理论计算进行了用于研究的氢(H 2)siligraphene的吸附性能(碳化硅8),原始和装饰有选择的碱(锂,钠,和钾)和碱土(铍,镁,和Ca)金属。我们发现,即使在高掺杂浓度(SiC 8 Me 2)下,除Mg和Be之外,所有考虑的金属(Me)也会通过将大部分价电子提供给SiC 8而牢固地与SiC 8结合。从头算分子动力学(AIMD)模拟证实了SiC 8 Me 2的热稳定性(Me = Li,Na,K,Ca)在300 K时。我们显示,掺杂Li,Na和Ca的SiC 8吸附了多个H 2分子,其结合能(E bind)比原始能量强至少两倍。碳化硅8(Ë原始绑定= -70兆电子伏每股H 2)。总的来说,每个金属原子上SiC 8 Li 2和SiC 8 Ca 2分别吸附两个和四个H 2分子,其中E结合在有效吸附/解吸过程所需的范围内。SiC 8的重量密度Li 2和SiC 8 Ca 2分别为5.5 wt%和7.3 wt%,超过了美国能源部2025年的5.5 wt%的目标。估计的H 2解吸温度基本上超过了液氮的沸点,证实了轻金属装饰的SiC 8作为H 2存储的有前途的材料的潜力。
更新日期:2020-09-14
中文翻译:

在选择金属装饰后阐明二维硅单晶(SiC8)单层的储氢特性
与色散校正密度泛函理论计算进行了用于研究的氢(H 2)siligraphene的吸附性能(碳化硅8),原始和装饰有选择的碱(锂,钠,和钾)和碱土(铍,镁,和Ca)金属。我们发现,即使在高掺杂浓度(SiC 8 Me 2)下,除Mg和Be之外,所有考虑的金属(Me)也会通过将大部分价电子提供给SiC 8而牢固地与SiC 8结合。从头算分子动力学(AIMD)模拟证实了SiC 8 Me 2的热稳定性(Me = Li,Na,K,Ca)在300 K时。我们显示,掺杂Li,Na和Ca的SiC 8吸附了多个H 2分子,其结合能(E bind)比原始能量强至少两倍。碳化硅8(Ë原始绑定= -70兆电子伏每股H 2)。总的来说,每个金属原子上SiC 8 Li 2和SiC 8 Ca 2分别吸附两个和四个H 2分子,其中E结合在有效吸附/解吸过程所需的范围内。SiC 8的重量密度Li 2和SiC 8 Ca 2分别为5.5 wt%和7.3 wt%,超过了美国能源部2025年的5.5 wt%的目标。估计的H 2解吸温度基本上超过了液氮的沸点,证实了轻金属装饰的SiC 8作为H 2存储的有前途的材料的潜力。


























 京公网安备 11010802027423号
京公网安备 11010802027423号