当前位置:
X-MOL 学术
›
J. Chin. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enhancement of the biochemical activity of some market antibiotics by chemical modification: Synthesis, characterization, and biochemical evaluation
Journal of the Chinese Chemical Society ( IF 1.8 ) Pub Date : 2020-09-14 , DOI: 10.1002/jccs.202000158 Abbas M. Abbas 1 , Sara R. Fisal 1 , Adel S. Orabi 1
Journal of the Chinese Chemical Society ( IF 1.8 ) Pub Date : 2020-09-14 , DOI: 10.1002/jccs.202000158 Abbas M. Abbas 1 , Sara R. Fisal 1 , Adel S. Orabi 1
Affiliation
|
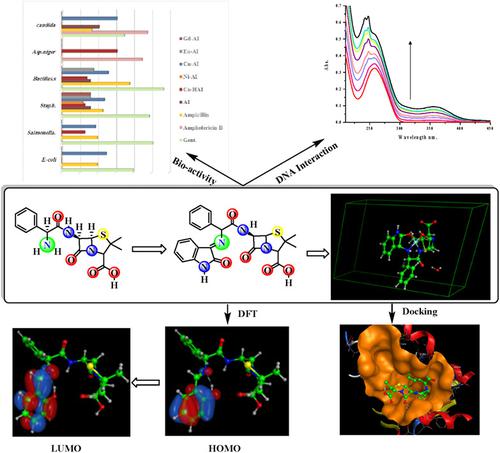
The reaction of isatin with the Ampicillin gave the new compound: (6R)‐3,3‐dimethyl‐7‐oxo‐6‐(2‐(([E]‐2‐oxoindolin‐3‐ylidene)amino)‐2‐phenylacetamido)‐4‐thia‐1‐azabicyclo[3.2.0]hept ‐ane‐2‐carboxylic acid (HAI). The new complexes derived from HAI and Co(II), Ni(II), Cu(II), Eu(III), and Gd(III) were obtained in pure form. The obtained compounds were characterized by elemental analysis, FTIR, UV–Vis, Fluorescence, 1HNMR, Mass spectra, DTA, TGA, Magnetic susceptibility, X‐ray, AAS, and the conductivity of 0.001 M in DMSO. The obtained data indicated the formation of the target complexes: [Co(HAI)(H2O)(NO3)]NO3.4H2O, [Ni(AI)(H2O)2]Cl.2H2O, [Cu(AI)]Cl.H2O, [Eu(AI)(H2O)Cl]Cl.5H2O and [Gd(AI)(H2O)(NO3)]NO3.3H2O. The ligation sites were predicted from the guide of the FTIR and thermal analysis meanwhile the stereochemistry was proved by the UV–Vis and magnetic moment. Co(II) and Ni(II) gave an octahedral structure while Cu(II) gave a square planar form. Molecular modeling, molecular mechanics, and DFT calculations were carried out for the synthesized compounds. The active lone pair and surface properties were obtained and discussed in the silico level. The x‐ray analysis indicates the nanoparticle behavior of the Cu‐AI complex with a monoclinic structure. The interactions of the synthesized complexes with FM‐DNA moiety were investigated through spectrometric titration (UV–vis. spectra) and by using fluorescence spectroscopy. The modes and binding affinities were evaluated and discussed using Benesi–Hildebrand method. Antimicrobial activities of the synthesized compounds have been screened using the disc diffusion method. HAI and Cu‐AI gave activity exceeded the Ampicillin. The docking work was carried using the targeting protein of Escherichia coli FabH (PDB code: 1HNJ). The obtained binding energy was compared and discussed in terms of the in vitro studies.
中文翻译:

通过化学修饰增强某些市场抗生素的生化活性:合成,表征和生化评估
Isatin与氨苄青霉素的反应产生了新化合物:(6R)-3,3-二甲基-7-氧代-6-(2-(([[[E] -2-氧代吲哚-3-3-亚萘基)氨基]氨基] -2-苯乙酰胺基)-4-硫杂-1-氮杂双环[3.2.0]庚烷-2-羧酸(HAI)。以纯净形式获得了源自HAI和Co(II),Ni(II),Cu(II),Eu(III)和Gd(III)的新络合物。通过元素分析,FTIR,UV-Vis,荧光,1 HNMR,质谱,DTA,TGA,磁化率,X射线,AAS和DMSO中0.001 M的电导率对所得化合物进行表征。所获得的数据表明了目标络合物的形成:[Co(HAI)(H 2 O)(NO 3)] NO 3 .4H 2 O,[Ni(Al)(H 2 O)2 ] Cl.2H 2O,[Cu(Al)] Cl.H 2 O,[Eu(Al)(H 2 O)Cl] Cl.5H 2 O和[Gd(Al)(H 2 O)(NO 3)] NO 3。 3小时2O.结合位点是根据FTIR和热分析指南预测的,同时立体化学由UV-Vis和磁矩证明。Co(II)和Ni(II)呈现八面体结构,而Cu(II)呈现方形平面形式。对合成的化合物进行了分子建模,分子力学和DFT计算。获得了活性孤对和表面性质并在计算机水平上进行了讨论。X射线分析表明具有单斜晶结构的Cu-AI复合物的纳米颗粒行为。通过光谱滴定(UV-可见光谱)和使用荧光光谱法研究了合成的复合物与FM-DNA部分的相互作用。使用Benesi–Hildebrand方法评估和讨论了模式和结合亲和力。已使用盘扩散法筛选了合成化合物的抗菌活性。HAI和Cu-AI的活性超过了氨苄西林。对接工作是使用靶向蛋白大肠杆菌FabH(PDB代码:1HNJ)。比较获得的结合能,并根据体外研究进行讨论。
更新日期:2020-09-14
中文翻译:

通过化学修饰增强某些市场抗生素的生化活性:合成,表征和生化评估
Isatin与氨苄青霉素的反应产生了新化合物:(6R)-3,3-二甲基-7-氧代-6-(2-(([[[E] -2-氧代吲哚-3-3-亚萘基)氨基]氨基] -2-苯乙酰胺基)-4-硫杂-1-氮杂双环[3.2.0]庚烷-2-羧酸(HAI)。以纯净形式获得了源自HAI和Co(II),Ni(II),Cu(II),Eu(III)和Gd(III)的新络合物。通过元素分析,FTIR,UV-Vis,荧光,1 HNMR,质谱,DTA,TGA,磁化率,X射线,AAS和DMSO中0.001 M的电导率对所得化合物进行表征。所获得的数据表明了目标络合物的形成:[Co(HAI)(H 2 O)(NO 3)] NO 3 .4H 2 O,[Ni(Al)(H 2 O)2 ] Cl.2H 2O,[Cu(Al)] Cl.H 2 O,[Eu(Al)(H 2 O)Cl] Cl.5H 2 O和[Gd(Al)(H 2 O)(NO 3)] NO 3。 3小时2O.结合位点是根据FTIR和热分析指南预测的,同时立体化学由UV-Vis和磁矩证明。Co(II)和Ni(II)呈现八面体结构,而Cu(II)呈现方形平面形式。对合成的化合物进行了分子建模,分子力学和DFT计算。获得了活性孤对和表面性质并在计算机水平上进行了讨论。X射线分析表明具有单斜晶结构的Cu-AI复合物的纳米颗粒行为。通过光谱滴定(UV-可见光谱)和使用荧光光谱法研究了合成的复合物与FM-DNA部分的相互作用。使用Benesi–Hildebrand方法评估和讨论了模式和结合亲和力。已使用盘扩散法筛选了合成化合物的抗菌活性。HAI和Cu-AI的活性超过了氨苄西林。对接工作是使用靶向蛋白大肠杆菌FabH(PDB代码:1HNJ)。比较获得的结合能,并根据体外研究进行讨论。


























 京公网安备 11010802027423号
京公网安备 11010802027423号