当前位置:
X-MOL 学术
›
J. Am. Coll. Cardiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Trial Design Principles for Patients at High Bleeding Risk Undergoing PCI
Journal of the American College of Cardiology ( IF 24.0 ) Pub Date : 2020-09-01 , DOI: 10.1016/j.jacc.2020.06.085 Davide Capodanno 1 , Marie-Claude Morice 2 , Dominick J Angiolillo 3 , Deepak L Bhatt 4 , Robert A Byrne 5 , Roisin Colleran 6 , Thomas Cuisset 7 , Donald Cutlip 8 , Pedro Eerdmans 9 , John Eikelboom 10 , Andrew Farb 11 , C Michael Gibson 12 , John Gregson 13 , Michael Haude 14 , Stefan K James 15 , Hyo-Soo Kim 16 , Takeshi Kimura 17 , Akihide Konishi 18 , Martin B Leon 19 , P F Adrian Magee 11 , Yoshiaki Mitsutake 18 , Darren Mylotte 20 , Stuart J Pocock 13 , Sunil V Rao 21 , Ernest Spitzer 22 , Norman Stockbridge 11 , Marco Valgimigli 23 , Olivier Varenne 24 , Ute Windhovel 2 , Mitchel W Krucoff 25 , Philip Urban 26 , Roxana Mehran 27
Journal of the American College of Cardiology ( IF 24.0 ) Pub Date : 2020-09-01 , DOI: 10.1016/j.jacc.2020.06.085 Davide Capodanno 1 , Marie-Claude Morice 2 , Dominick J Angiolillo 3 , Deepak L Bhatt 4 , Robert A Byrne 5 , Roisin Colleran 6 , Thomas Cuisset 7 , Donald Cutlip 8 , Pedro Eerdmans 9 , John Eikelboom 10 , Andrew Farb 11 , C Michael Gibson 12 , John Gregson 13 , Michael Haude 14 , Stefan K James 15 , Hyo-Soo Kim 16 , Takeshi Kimura 17 , Akihide Konishi 18 , Martin B Leon 19 , P F Adrian Magee 11 , Yoshiaki Mitsutake 18 , Darren Mylotte 20 , Stuart J Pocock 13 , Sunil V Rao 21 , Ernest Spitzer 22 , Norman Stockbridge 11 , Marco Valgimigli 23 , Olivier Varenne 24 , Ute Windhovel 2 , Mitchel W Krucoff 25 , Philip Urban 26 , Roxana Mehran 27
Affiliation
|
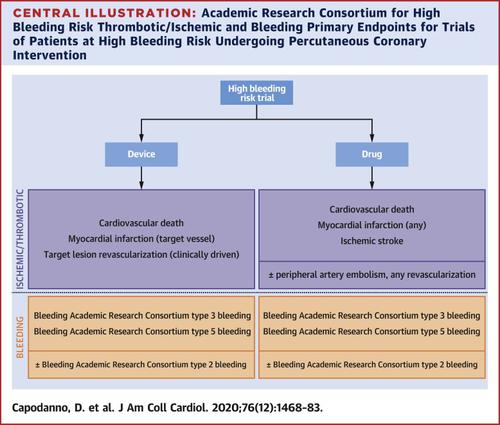
Investigating the balance of risk for thrombotic and bleeding events after percutaneous coronary intervention (PCI) is especially relevant for patients at high bleeding risk (HBR). The Academic Research Consortium for HBR recently proposed a consensus definition in an effort to standardize the patient population included in HBR trials. The aim of this consensus-based document, the second initiative from the Academic Research Consortium for HBR, is to propose recommendations to guide the design of clinical trials of devices and drugs in HBR patients undergoing PCI. The authors discuss the designs of trials in HBR patients undergoing PCI and various aspects of trial design specific to HBR patients, including target populations, intervention and control groups, primary and secondary outcomes, and timing of endpoint reporting.
中文翻译:

接受 PCI 的高出血风险患者的试验设计原则
调查经皮冠状动脉介入治疗 (PCI) 后血栓形成和出血事件风险的平衡对于高出血风险 (HBR) 患者尤其重要。HBR 学术研究联盟最近提出了一个共识定义,以努力使 HBR 试验中包含的患者群体标准化。这份基于共识的文件是 HBR 学术研究联盟的第二个倡议,其目的是提出建议,以指导在接受 PCI 的 HBR 患者中设计设备和药物的临床试验。作者讨论了接受 PCI 的 HBR 患者的试验设计以及针对 HBR 患者的试验设计的各个方面,包括目标人群、干预组和对照组、主要和次要结果以及终点报告的时间安排。
更新日期:2020-09-01
中文翻译:

接受 PCI 的高出血风险患者的试验设计原则
调查经皮冠状动脉介入治疗 (PCI) 后血栓形成和出血事件风险的平衡对于高出血风险 (HBR) 患者尤其重要。HBR 学术研究联盟最近提出了一个共识定义,以努力使 HBR 试验中包含的患者群体标准化。这份基于共识的文件是 HBR 学术研究联盟的第二个倡议,其目的是提出建议,以指导在接受 PCI 的 HBR 患者中设计设备和药物的临床试验。作者讨论了接受 PCI 的 HBR 患者的试验设计以及针对 HBR 患者的试验设计的各个方面,包括目标人群、干预组和对照组、主要和次要结果以及终点报告的时间安排。


























 京公网安备 11010802027423号
京公网安备 11010802027423号