当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Protecting‐Group‐Free Total Synthesis and Biological Investigation of Cabucine Oxindole A†
Chinese Journal of Chemistry ( IF 5.4 ) Pub Date : 2020-09-13 , DOI: 10.1002/cjoc.202000460 Shengling Xie 1 , Chengqing Ning 1 , Qingzhen Yu 1 , Jieping Hou 1 , Jing Xu 1
Chinese Journal of Chemistry ( IF 5.4 ) Pub Date : 2020-09-13 , DOI: 10.1002/cjoc.202000460 Shengling Xie 1 , Chengqing Ning 1 , Qingzhen Yu 1 , Jieping Hou 1 , Jing Xu 1
Affiliation
|
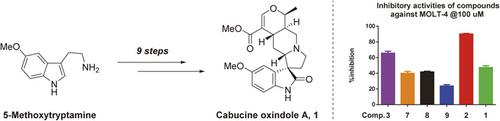
Owing to their challenging structures and promising biological profiles, spirooxindole alkaloids have long attracted much attention from the synthetic community. Herein, we wish to describe a concise, protecting‐group‐free total synthesis of cabucine oxindole A, a putative natural spirooxindole alkaloid and a possible biosynthetic congener of cabucine and palmirine. Key transformations of our approach include a one‐step, organocatalytic and enantioselective construction of the spiro[pyrrolidine‐3,3’‐oxindole] moiety and a Korte rearrangement to furnish the final dihydropyran motif. Biological investigation of 1 and its synthetic intermediates revealed lactone 2 as a mild MOLT‐4 and MCF7 cell line inhibitor.
中文翻译:

卡维辛氧吲哚A†的无保护基团的全合成和生物学研究
由于螺环吲哚生物碱具有挑战性的结构和有希望的生物学特性,长期以来一直引起合成界的广泛关注。本文中,我们希望描述一种简洁,无保护基团的卡布西诺羟吲哚A,一种推定的天然螺环氧吲哚生物碱以及卡布西因和棕榈酸的可能的生物合成同类物。我们方法的关键转变包括螺[吡咯烷-3,3'-氧吲哚]部分的一步有机催化和对映选择性构建,以及提供最终的二氢吡喃基序的Korte重排。对1及其合成中间体的生物学研究表明,内酯2是轻度的MOLT-4和MCF7细胞系抑制剂。
更新日期:2020-09-13
中文翻译:

卡维辛氧吲哚A†的无保护基团的全合成和生物学研究
由于螺环吲哚生物碱具有挑战性的结构和有希望的生物学特性,长期以来一直引起合成界的广泛关注。本文中,我们希望描述一种简洁,无保护基团的卡布西诺羟吲哚A,一种推定的天然螺环氧吲哚生物碱以及卡布西因和棕榈酸的可能的生物合成同类物。我们方法的关键转变包括螺[吡咯烷-3,3'-氧吲哚]部分的一步有机催化和对映选择性构建,以及提供最终的二氢吡喃基序的Korte重排。对1及其合成中间体的生物学研究表明,内酯2是轻度的MOLT-4和MCF7细胞系抑制剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号