当前位置:
X-MOL 学术
›
Appl. Organomet. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Optical properties, structural, and DFT studies of Pd(II) complexes with 1‐phenyl‐1H‐tetrazol‐5‐thiol, X‐ray crystal structure of [Pd(κ1‐S‐ptt)2(κ2‐dppe)] complex
Applied Organometallic Chemistry ( IF 3.9 ) Pub Date : 2020-09-12 , DOI: 10.1002/aoc.5996 Ahmed S.M. Al‐Janabi 1 , Usama'a A.Y. Al‐Samra 2 , Eman A. Othman 2 , T.A. Yousef 3, 4
Applied Organometallic Chemistry ( IF 3.9 ) Pub Date : 2020-09-12 , DOI: 10.1002/aoc.5996 Ahmed S.M. Al‐Janabi 1 , Usama'a A.Y. Al‐Samra 2 , Eman A. Othman 2 , T.A. Yousef 3, 4
Affiliation
|
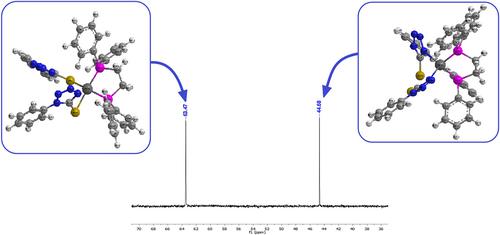
Seven tetrazole‐thione complexes, [Pd2(κ2‐ptt)4](1), trans‐[Pd(k1‐S‐ptt)2(PPh3)2] (2), trans‐[Pd(k1‐S‐ptt)2(SPPh3)2] (3), trans‐[Pd(k1‐S‐ptt)2(OPPh3)2] (4), [Pd(k1‐N‐ptt)2(k2‐dppe)] (5a), [Pd(k1‐S‐ptt)2(k2‐dppe)] (5b), [Pd(k1‐S‐ptt)2(k2‐dppeS2)] (6), and [Pd(k1‐S‐ptt)2(k2‐dppeO2)] (7), were prepared from 1‐phenyl‐1H‐tetrazole‐5‐thiol (Hptt), with substituted phosphines. These complexes were investigated by CHNS analysis; infrared (IR), nuclear magnetic resonance (NMR) (1H and 31P), and ultraviolet–visible (UV–Vis) spectroscopy; and single‐crystal X‐ray data for 5b. In Complex 1, the ptt− ligand adopted μ2‐ k‐N, k‐S bridging mode to afford a dimeric complex, whereas in Complexes 2–4, 6, and 7, the ptt− was covalently coordinated via sulfur atom of the thiol group as a solo product. In contrast, in Complex 5, the ptt− ligand was bonded in a monodentate fashion through a deprotonated tetrazole ring nitrogen atom in isomer 5a or via a thiolato sulfur atom in isomer 5b. These linkage isomers were clearly shown in the 31P‐{1H} NMR. To explain the adoption of the ligand binding modes in Complexes 5a and 5b, geometry optimization calculations were carried out on two isomers. Very small differences of all molecular parameters were found between 5a and 5b isomers. This confirms the reason for obtaining two isomers. Also, theoretical studies are made for all compounds, and excellent agreement is obtained with experimental data. The direct band gap (Eg) values are equal to 2.88, 2.85, and 2.45 eV for Complexes 1, 2, and 4, respectively, revealing a semiconductor nature. The inhibition activity of Complexes 1–3, 5, and 8 were evaluated versus the growth of four types of bacteria in vitro. The complexes showed a good activity compared with free ligand and a standard antibiotic.
中文翻译:

具有1-苯基-1H-四唑-5-硫醇的Pd(II)配合物的光学性质,结构和DFT研究,[Pd(κ1-S-ptt)2(κ2-dppe)]配合物的X射线晶体结构
七四唑硫酮络合物,[钯2(κ 2 -ptt)4 ](1),反式- [钯(ķ 1 -小号-ptt)2(PPH 3)2 ](2),反式- [钯(ķ 1 - S ptt)2(SPPh 3)2 ](3),反-[Pd(k 1 - S ptt)2(OPPh 3)2 ](4),[Pd(k 1 - N -ptt)2(k 2 -dppe)](5a),[Pd(k 1 - S -ptt)2(k 2 -dppe)](5b),[Pd(k 1 - S -ptt)2(k 2 -dppeS 2)](6)和[Pd(k 1 - S- ptt)2(k 2 -dppeO 2)](7)由1-苯基-1H-四唑-5-硫醇(Hptt)与取代的膦制备。通过CHNS分析研究了这些配合物。红外(IR),核磁共振(NMR)(1 H和31 P)和紫外可见(UV-Vis)光谱;和5b的单晶X射线数据。在复杂1中,PTT -配体采用μ 2 - ķ - ñ,ķ - š桥接模式,得到的二聚体复合物,而在配合物2-4,6,和7中,PTT -通过硫醇基团的硫原子作为单独产物共价配位。与此相反,在配合物5中,PTT -配位体通过在异构体中的脱质子四唑环氮原子键合在单齿的方式5A或通过在异构体thiolato硫原子5b中。在31 P- { 1 H} NMR中清楚地显示了这些键合异构体。为了解释在配合物5a和5b中采用配体结合模式,对两个异构体进行了几何优化计算。在5a和5b之间发现所有分子参数的差异很小异构体。这证实了获得两种异构体的原因。同样,对所有化合物进行了理论研究,并与实验数据获得了很好的一致性。直接带隙(Eg)值等于2.88,2.85,和2.45电子伏特为配合物1,2,和4分别,露出半导体性质。配合物的抑制活性1-3,5,和8进行了评价对四种类型的体外细菌的生长。与游离配体和标准抗生素相比,该复合物显示出良好的活性。
更新日期:2020-11-06
中文翻译:

具有1-苯基-1H-四唑-5-硫醇的Pd(II)配合物的光学性质,结构和DFT研究,[Pd(κ1-S-ptt)2(κ2-dppe)]配合物的X射线晶体结构
七四唑硫酮络合物,[钯2(κ 2 -ptt)4 ](1),反式- [钯(ķ 1 -小号-ptt)2(PPH 3)2 ](2),反式- [钯(ķ 1 - S ptt)2(SPPh 3)2 ](3),反-[Pd(k 1 - S ptt)2(OPPh 3)2 ](4),[Pd(k 1 - N -ptt)2(k 2 -dppe)](5a),[Pd(k 1 - S -ptt)2(k 2 -dppe)](5b),[Pd(k 1 - S -ptt)2(k 2 -dppeS 2)](6)和[Pd(k 1 - S- ptt)2(k 2 -dppeO 2)](7)由1-苯基-1H-四唑-5-硫醇(Hptt)与取代的膦制备。通过CHNS分析研究了这些配合物。红外(IR),核磁共振(NMR)(1 H和31 P)和紫外可见(UV-Vis)光谱;和5b的单晶X射线数据。在复杂1中,PTT -配体采用μ 2 - ķ - ñ,ķ - š桥接模式,得到的二聚体复合物,而在配合物2-4,6,和7中,PTT -通过硫醇基团的硫原子作为单独产物共价配位。与此相反,在配合物5中,PTT -配位体通过在异构体中的脱质子四唑环氮原子键合在单齿的方式5A或通过在异构体thiolato硫原子5b中。在31 P- { 1 H} NMR中清楚地显示了这些键合异构体。为了解释在配合物5a和5b中采用配体结合模式,对两个异构体进行了几何优化计算。在5a和5b之间发现所有分子参数的差异很小异构体。这证实了获得两种异构体的原因。同样,对所有化合物进行了理论研究,并与实验数据获得了很好的一致性。直接带隙(Eg)值等于2.88,2.85,和2.45电子伏特为配合物1,2,和4分别,露出半导体性质。配合物的抑制活性1-3,5,和8进行了评价对四种类型的体外细菌的生长。与游离配体和标准抗生素相比,该复合物显示出良好的活性。

























 京公网安备 11010802027423号
京公网安备 11010802027423号