International Journal of Hydrogen Energy ( IF 7.2 ) Pub Date : 2020-09-12 , DOI: 10.1016/j.ijhydene.2020.08.145 Anant Prakash Pandey , Ashish Bhatnagar , Vivek Shukla , Pawan K. Soni , Sweta Singh , Satish K. Verma , M. Shaneeth , V. Sekkar , O.N. Srivastava
|
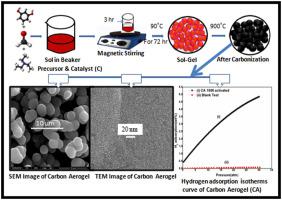
In this paper, we report here the hydrogen storage capacity of activated carbon aerogel synthesized by ambient pressure drying using a new catalyst. The carbon aerogel (CA) has been synthesized by the sol-gel method using resorcinol (R) and formaldehyde (F). For drying of RF wet gel instead of expensive and unsafe supercritical process, we have used ambient pressure drying. To avoid shrinkage which may occur due to this mode of drying, instead of usual catalyst (C): Na2CO3, organic catalyst triethylamine (TEA), which is known to be a condensing agent has been used. In order to find out the effect of change of R/C ratio on hydrogen sorption, three different R/C namely CA 1000, CA 2000, and CA 3000 were taken. Structural and microstructural details have been studied employing XRD, SEM, TEM, nitrogen adsorption, FTIR, and Raman spectroscopy. TEM and nitrogen adsorption studies have revealed that aerogel with R/C 1000 exhibits a higher degree of micropore density. The hydrogen storage capacities for all R/C ratios have been determined. It has been found that carbon aerogel (CA) with R/C = 1000, exhibits the highest hydrogen adsorption capacity out of the three aerogels. At liquid nitrogen temperature, the hydrogen storage capacity of aerogel with R/C = 1000 for the as-synthesized and activated carbons have been found to be 4.00 wt % and 4.80 wt %. A viable reason for the occurrence of high hydrogen storage capacity at liquid nitrogen temperature for aerogel with R/C = 1000 has been put forward.
中文翻译:

新催化剂三乙胺常压干燥合成碳气凝胶的储氢性能
在本文中,我们在这里报告了使用新催化剂通过环境压力干燥合成的活性炭气凝胶的储氢能力。使用间苯二酚(R)和甲醛(F)通过溶胶-凝胶法合成了碳气凝胶(CA)。为了干燥RF湿凝胶,而不是昂贵且不安全的超临界过程,我们使用了常压干燥。为了避免由于这种干燥方式而可能发生的收缩,而不是通常的催化剂(C):Na 2 CO 3已知使用有机催化剂三乙胺(TEA)作为缩合剂。为了找出R / C比的变化对氢吸附的影响,采用了三种不同的R / C,即CA 1000,CA 2000和CA 3000。已经使用XRD,SEM,TEM,氮吸附,FTIR和拉曼光谱研究了结构和微观结构细节。TEM和氮吸附研究表明,R / C 1000的气凝胶具有较高的微孔密度。已确定所有R / C比的储氢容量。已经发现,R / C = 1000的碳气凝胶(CA)在三种气凝胶中均表现出最高的氢吸附能力。在液氮温度下 对于合成和活性炭而言,R / C = 1000的气凝胶的储氢能力为4.00 wt%和4.80 wt%。提出了在R / C = 1000的气凝胶中液氮温度下储氢能力高的可行原因。



























 京公网安备 11010802027423号
京公网安备 11010802027423号