Aquatic Toxicology ( IF 4.5 ) Pub Date : 2020-09-12 , DOI: 10.1016/j.aquatox.2020.105627 Arnab Seth 1 , Kunal Roy 1
|
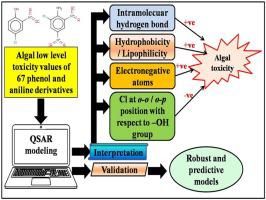
The deposition of different types of phenol and aniline derivatives in the aquatic environment leads to toxicity to living organisms. Under such condition, evaluation of these toxicants is very much important. Due to non-availability of sufficient experimental data as well as sufficient number of Quantitative Structure-Activity Relationship (QSAR) models for the low level toxicity values for such pollutants, we have employed here the partial least squares (PLS) regression for the development of robust and predictive QSAR models using low level toxicity values against algal species. Here, we have used both Extended Topochemical Atom (ETA) and non-ETA indices as 2D descriptors for model development. The statistical validation parameters ensure the robustness and the predictivity of the developed models. From the insights of the final PLS models, it can be concluded that presence of nitro groups (in the ortho position to phenolic hydroxyl group increasing intramolecular hydrogen bonding capacity), presence of chlorine substituents (influencing lipophilicity) especially at the para position, oxygen and nitrogen at the topological distance three, aliphatic side chain (contributing to hydrophobicity), molecules with large size atoms and higher molecular bulk will increase the toxicity towards the algal species. On the other hand, the phenol ring without any substituent or with a polar substituent (like amino group), presence of chlorine at ortho-ortho or ortho-para position, absence of nitro group, presence of chlorine and oxygen at the topological distance three, presence of lower number of aliphatic groups will decrease the toxic effect towards the algal species.
中文翻译:

使用2D描述符对不同苯酚和苯胺衍生物的藻类低水平毒性值进行QSAR建模。
在水生环境中沉积不同类型的苯酚和苯胺衍生物会导致对生物体的毒性。在这种条件下,对这些有毒物质的评估非常重要。由于没有足够的实验数据以及足够数量的定量结构-活性关系(QSAR)模型来针对此类污染物的低水平毒性值,我们在这里采用偏最小二乘(PLS)回归方法开发污染物。使用针对藻类的低水平毒性值的可靠且可预测的QSAR模型。在这里,我们同时使用扩展拓扑化学原子(ETA)和非ETA索引作为模型开发的2D描述子。统计验证参数可确保所开发模型的鲁棒性和可预测性。从最终的PLS模型的见解中,酚羟基的邻位增加了分子内氢键的能力),尤其是在对位处存在氯取代基(影响亲脂性),在拓扑距离上存在氧和氮3,脂族侧链(有助于疏水性),具有大尺寸原子的分子较高的分子体积将增加对藻类的毒性。另一方面,没有任何取代基或带有极性取代基(如氨基)的苯酚环,在邻-邻或邻-对 位置,不存在硝基,在拓扑距离3处存在氯和氧,较少数量的脂族基团的存在将降低对藻类的毒性作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号