当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Immobilizing Pertechnetate in Ettringite via Sulfate Substitution.
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2020-09-10 , DOI: 10.1021/acs.est.0c03119 Sarah A Saslow 1 , Sebastien N Kerisit 2 , Tamas Varga 3 , Sebastian T Mergelsberg 2 , Claire L Corkhill 4 , Michelle M V Snyder 1 , Nancy M Avalos 1 , Antonia S Yorkshire 4 , Daniel J Bailey 4 , Jarrod Crum 1 , R Matthew Asmussen 1
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2020-09-10 , DOI: 10.1021/acs.est.0c03119 Sarah A Saslow 1 , Sebastien N Kerisit 2 , Tamas Varga 3 , Sebastian T Mergelsberg 2 , Claire L Corkhill 4 , Michelle M V Snyder 1 , Nancy M Avalos 1 , Antonia S Yorkshire 4 , Daniel J Bailey 4 , Jarrod Crum 1 , R Matthew Asmussen 1
Affiliation
|
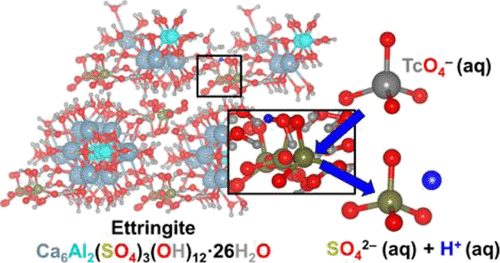
Technetium-99 immobilization in low-temperature nuclear waste forms often relies on additives that reduce environmentally mobile pertechnetate (TcO4–) to insoluble Tc(IV) species. However, this is a short-lived solution unless reducing conditions are maintained over the hazardous life cycle of radioactive wastes (some ∼10,000 years). Considering recent experimental observations, this work explores how rapid formation of ettringite [Ca6Al2(SO4)3(OH)12·26(H2O)], a common mineral formed in cementitious waste forms, may be used to directly immobilize TcO4–. Results from ab initio molecular dynamics (AIMD) simulations and solid-phase characterization techniques, including synchrotron X-ray absorption, fluorescence, and diffraction methods, support successful incorporation of TcO4– into the ettringite crystal structure via sulfate substitution when synthesized by aqueous precipitation methods. One sulfate and one water are replaced with one TcO4– and one OH– during substitution, where Ca2+-coordinated water near the substitution site is deprotonated to form OH– for charge compensation upon TcO4– substitution. Furthermore, AIMD calculations support favorable TcO4– substitution at the SO42– site in ettringite rather than gypsum (CaSO4·2H2O, formed as a secondary mineral phase) by at least 0.76 eV at 298 K. These results are the first of their kind to suggest that ettringite may contribute to TcO4– immobilization and the overall lifetime performance of cementitious waste forms.
中文翻译:

通过硫酸盐置换将高tech酸酯固定在钙矾石中。
将Technetium-99固定在低温核废料中通常依赖于将环境可移动的高mobile酸盐(TcO 4 –)还原为不溶性Tc(IV)物质的添加剂。但是,这是一个短暂的解决方案,除非在放射性废物的危险生命周期(约10,000年)内保持降低条件。考虑到最近的实验观察,这项工作探索了如何快速形成钙矾石[Ca 6 Al 2(SO 4)3(OH)12 ·26(H 2 O)],这是一种以水泥废物形式形成的常见矿物,可用于直接形成钙钛矿。固定TcO 4 –。从AB结果算分子动力学(AIMD)模拟和固相表征技术,包括同步辐射X射线的吸收,荧光和衍射法,TCO的支撑成功掺入4 -成经由硫酸盐替代的钙矾石晶体结构通过水性沉淀合成时方法。一个硫酸盐和一种水被替换为一个TCO 4 -和一个OH -取代,其中C期间2+的取代部位附近配位的水去质子化,以形成OH -用于在TCO电荷补偿4 -置换。此外,AIMD计算支持有利的TcO 4 –在298 K时至少在钙矾石中的SO 4 2–处而不是石膏(作为次生矿物相形成的CaSO 4 ·2H 2 O)上被0.76 eV取代。这些结果首次表明钙矾石可能有助于TcO 4 –固结性废物形式的固定化和整个生命周期的表现。
更新日期:2020-11-03
中文翻译:

通过硫酸盐置换将高tech酸酯固定在钙矾石中。
将Technetium-99固定在低温核废料中通常依赖于将环境可移动的高mobile酸盐(TcO 4 –)还原为不溶性Tc(IV)物质的添加剂。但是,这是一个短暂的解决方案,除非在放射性废物的危险生命周期(约10,000年)内保持降低条件。考虑到最近的实验观察,这项工作探索了如何快速形成钙矾石[Ca 6 Al 2(SO 4)3(OH)12 ·26(H 2 O)],这是一种以水泥废物形式形成的常见矿物,可用于直接形成钙钛矿。固定TcO 4 –。从AB结果算分子动力学(AIMD)模拟和固相表征技术,包括同步辐射X射线的吸收,荧光和衍射法,TCO的支撑成功掺入4 -成经由硫酸盐替代的钙矾石晶体结构通过水性沉淀合成时方法。一个硫酸盐和一种水被替换为一个TCO 4 -和一个OH -取代,其中C期间2+的取代部位附近配位的水去质子化,以形成OH -用于在TCO电荷补偿4 -置换。此外,AIMD计算支持有利的TcO 4 –在298 K时至少在钙矾石中的SO 4 2–处而不是石膏(作为次生矿物相形成的CaSO 4 ·2H 2 O)上被0.76 eV取代。这些结果首次表明钙矾石可能有助于TcO 4 –固结性废物形式的固定化和整个生命周期的表现。



























 京公网安备 11010802027423号
京公网安备 11010802027423号