当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Lessons about Protein Folding and Binding from Archetypal Folds.
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2020-09-11 , DOI: 10.1021/acs.accounts.0c00322 Luis Alberto Campos 1, 2 , Mourad Sadqi 3, 4 , Victor Muñoz 3, 4, 5
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2020-09-11 , DOI: 10.1021/acs.accounts.0c00322 Luis Alberto Campos 1, 2 , Mourad Sadqi 3, 4 , Victor Muñoz 3, 4, 5
Affiliation
|
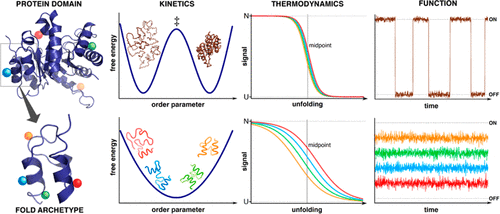
The function of proteins as biological nanomachines relies on their ability to fold into complex 3D structures, bind selectively to partners, and undergo conformational changes on cue. The native functional structures, and the rates of interconversion between conformational states (folded-unfolded, bound-free), are all encoded in the physical chemistry of their amino acid sequence. However, despite extensive research over decades, this code has proven difficult to fully crack, in terms of both prediction and understanding the molecular mechanisms at play.
中文翻译:

原型折叠中有关蛋白质折叠和结合的课程。
蛋白质作为生物纳米机器的功能依赖于它们折叠成复杂的3D结构,选择性地与伴侣结合以及在提示时发生构象变化的能力。天然功能结构以及构象状态之间的相互转化率(折叠,未折叠,无键)均在其氨基酸序列的物理化学中进行编码。然而,尽管进行了数十年的广泛研究,但从预测和理解起作用的分子机理两方面来看,很难证明完全破解该代码。
更新日期:2020-10-21
中文翻译:

原型折叠中有关蛋白质折叠和结合的课程。
蛋白质作为生物纳米机器的功能依赖于它们折叠成复杂的3D结构,选择性地与伴侣结合以及在提示时发生构象变化的能力。天然功能结构以及构象状态之间的相互转化率(折叠,未折叠,无键)均在其氨基酸序列的物理化学中进行编码。然而,尽管进行了数十年的广泛研究,但从预测和理解起作用的分子机理两方面来看,很难证明完全破解该代码。



























 京公网安备 11010802027423号
京公网安备 11010802027423号