当前位置:
X-MOL 学术
›
Appl. Organomet. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis, characterization, and evaluation of biological activities of new 4′‐substituted ruthenium (II) terpyridine complexes: Prospective anti‐inflammatory properties
Applied Organometallic Chemistry ( IF 3.9 ) Pub Date : 2020-09-11 , DOI: 10.1002/aoc.6024 Mohamed M. Elnagar 1 , Safia Samir 2 , Yasser M. Shaker 3 , Ayman A. Abdel‐Shafi 4 , Walid Sharmoukh 1 , Mohamed S. Abdel‐Aziz 5 , Khaled S. Abou‐El‐Sherbini 1
Applied Organometallic Chemistry ( IF 3.9 ) Pub Date : 2020-09-11 , DOI: 10.1002/aoc.6024 Mohamed M. Elnagar 1 , Safia Samir 2 , Yasser M. Shaker 3 , Ayman A. Abdel‐Shafi 4 , Walid Sharmoukh 1 , Mohamed S. Abdel‐Aziz 5 , Khaled S. Abou‐El‐Sherbini 1
Affiliation
|
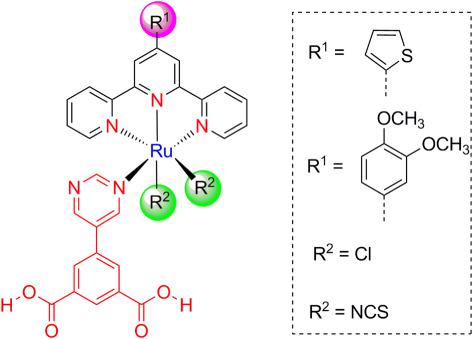
The synthesis and characterization of Ru (II) terpyridine complexes derived from 4′ functionalized 2,2′:6′,2″‐terpyridine (tpy) ligands are reported. The heteroleptic complexes comprise the synthesized ligands 4′‐(2‐thienyl)‐ 2,2′:6′,2″‐terpyridine) or (4′‐(3,4‐dimethoxyphenyl)‐2,2′:6′,2″‐terpyridine and (dimethyl 5‐(pyrimidin‐5‐yl)isophthalate). The new complexes [Ru(4′‐(2‐thienyl)‐2,2′:6′,2″‐terpyridine)(5‐(pyrimidin‐5‐yl)‐isophthalic acid)Cl2] (9), [Ru(4′‐(3,4‐dimethoxyphenyl)‐2,2′:6′,2″‐terpyridine)(5‐(pyrimidin‐5‐yl)‐isophthalic acid)Cl2] (10), and [Ru(4′‐(2‐thienyl)‐2,2′:6′,2″‐terpyridine)(5‐(pyrimidin‐5‐yl)‐isophthalic acid)(NCS)2] (11) were characterized by 1H‐ and 13C‐NMR spectroscopy, C, H, N, and S elemental analysis, UPLC‐ESI‐MS, TGA, FT‐IR, and UV‐Vis spectroscopy. The biological activities of the synthesized ligands and their Ru (II) complexes as anti‐inflammatory, antimicrobial, and anticancer agents were evaluated. Furthermore, the toxicity of the synthesized compounds was studied and compared with the standard drugs, namely, diclofenac potassium and ibuprofen, using hemolysis assay. The results indicated that the ligands and the complex 9 possess superior anti‐inflammatory activities inhibiting albumin denaturation (89.88–100%) compared with the standard drugs (51.5–88.37%) at a concentration of 500 μg g−1. These activities were related to the presence of the chelating N‐atoms in the ligands and the exchangeable chloro‐ groups in the complex. Moreover, the chloro‐ and thiophene groups in complex 9 produce a higher anticancer activity compared with its isothiocyanate derivative in the complex 11 and the 3,4‐dimethoxyphenyl moiety in complex 10. Considering the toxicity results, the synthesized ligands are nontoxic or far less toxic compared with the standard drugs and the metal complexes. Therefore, these newly synthesized compounds are promising anti‐inflammatory agents in addition to their moderate unique broad antimicrobial activity.
中文翻译:

新的4'-取代的钌(II)吡啶吡啶配合物的合成,表征和生物活性的评估:预期的抗炎特性
报道了衍生自4'功能化的2,2':6',2''-三联吡啶(tpy)配体的Ru(II)联吡啶复合物的合成和表征。杂配物复合物包含合成的配体4'-(2-噻吩基)-2,2':6',2''-叔吡啶)或(4'-(3,4-二甲氧基苯基)-2,2':6', 2″-叔吡啶和(5-(嘧啶-5-5-基)间苯二甲酸二甲酯)。新络合物[Ru(4'-(2-噻吩基)-2,2':6',2″-叔吡啶)(5- (嘧啶-5-基)-间苯二甲酸Cl 2 ](9),[Ru(4'-(3,4-二甲氧基苯基)-2-,2':6',2″-叔吡啶)(5-(嘧啶-5-基)-间苯二甲酸)Cl 2 ](10)和[Ru(4'-(2-噻吩基)-2,2':6',2″-吡啶基)(5-(嘧啶-5- yl)-间苯二甲酸(NCS)2 ](11)的特征为1H-和13 C-NMR光谱,C,H,N和S元素分析,UPLC-ESI-MS,TGA,FT-IR和UV-Vis光谱。评估了合成的配体及其Ru(II)复合物作为抗炎,抗微生物和抗癌剂的生物活性。此外,使用溶血测定法研究了合成化合物的毒性并将其与标准药物双氯芬酸钾和布洛芬进行了比较。结果表明,与标准药物(51.5–88.37%)相比,配体和复合物9在浓度为500μgg -1时具有抑制白蛋白变性的优异抗炎活性(89.88–100%)。。这些活性与配体中存在螯合的N原子和复合物中的可交换氯基有关。此外,与配合物11中的异硫氰酸酯衍生物和配合物10中的3,4-二甲氧基苯基部分相比,配合物9中的氯和噻吩基团具有更高的抗癌活性。考虑到毒性结果,与标准药物和金属配合物相比,合成的配体无毒或毒性低得多。因此,这些新合成的化合物除具有中等独特的广泛抗菌活性外,还有望成为抗炎药。
更新日期:2020-09-11
中文翻译:

新的4'-取代的钌(II)吡啶吡啶配合物的合成,表征和生物活性的评估:预期的抗炎特性
报道了衍生自4'功能化的2,2':6',2''-三联吡啶(tpy)配体的Ru(II)联吡啶复合物的合成和表征。杂配物复合物包含合成的配体4'-(2-噻吩基)-2,2':6',2''-叔吡啶)或(4'-(3,4-二甲氧基苯基)-2,2':6', 2″-叔吡啶和(5-(嘧啶-5-5-基)间苯二甲酸二甲酯)。新络合物[Ru(4'-(2-噻吩基)-2,2':6',2″-叔吡啶)(5- (嘧啶-5-基)-间苯二甲酸Cl 2 ](9),[Ru(4'-(3,4-二甲氧基苯基)-2-,2':6',2″-叔吡啶)(5-(嘧啶-5-基)-间苯二甲酸)Cl 2 ](10)和[Ru(4'-(2-噻吩基)-2,2':6',2″-吡啶基)(5-(嘧啶-5- yl)-间苯二甲酸(NCS)2 ](11)的特征为1H-和13 C-NMR光谱,C,H,N和S元素分析,UPLC-ESI-MS,TGA,FT-IR和UV-Vis光谱。评估了合成的配体及其Ru(II)复合物作为抗炎,抗微生物和抗癌剂的生物活性。此外,使用溶血测定法研究了合成化合物的毒性并将其与标准药物双氯芬酸钾和布洛芬进行了比较。结果表明,与标准药物(51.5–88.37%)相比,配体和复合物9在浓度为500μgg -1时具有抑制白蛋白变性的优异抗炎活性(89.88–100%)。。这些活性与配体中存在螯合的N原子和复合物中的可交换氯基有关。此外,与配合物11中的异硫氰酸酯衍生物和配合物10中的3,4-二甲氧基苯基部分相比,配合物9中的氯和噻吩基团具有更高的抗癌活性。考虑到毒性结果,与标准药物和金属配合物相比,合成的配体无毒或毒性低得多。因此,这些新合成的化合物除具有中等独特的广泛抗菌活性外,还有望成为抗炎药。


























 京公网安备 11010802027423号
京公网安备 11010802027423号