当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
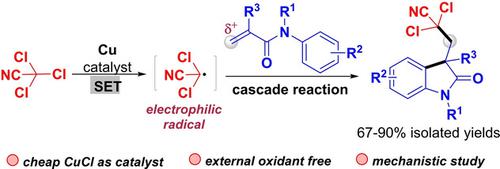
Synthesis of Dichlorocyanomethyl‐Functionalized Oxindoles by Cascade Reactions Initiated by Copper(I)‐Catalytically Generated Dichlorocyanomethyl Radical
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-09-10 , DOI: 10.1002/adsc.202000600 Fengrui Che 1 , Jiangbin Zhong 1 , Linhan Yu 1 , Chaopeng Ma 1 , Chunzheng Yu 1 , Maorong Wang 2 , Zhichen Hou 1 , Yuexia Zhang 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-09-10 , DOI: 10.1002/adsc.202000600 Fengrui Che 1 , Jiangbin Zhong 1 , Linhan Yu 1 , Chaopeng Ma 1 , Chunzheng Yu 1 , Maorong Wang 2 , Zhichen Hou 1 , Yuexia Zhang 1
Affiliation
|
An efficient and convenient protocol for synthesis of dichlorocyanomethyl‐functionalized oxindoles through the cascade reactions of dichlorocyanomethyl radical with N‐arylacrylamides is reported. The electrophilic dichlorocyanomethyl radical, which is generated from readily available trichloroacetonitrile via a copper‐catalyzed single‐electron transfer (SET) process, undergoes radical addition with the electron‐deficient C=C bonds of N‐arylacrylamides, followed by intramolecular cyclization, to provide dichlorocyanomethyl‐functionalized oxindoles. No external oxidant is needed in this transformation.
中文翻译:

铜(I)催化生成的二氯氰基甲基自由基引发的级联反应合成二氯氰基甲基官能化的吲哚
据报道,通过二氯氰基甲基自由基与N-芳基丙烯酰胺的级联反应,合成二氯氰基甲基官能化的吲哚的有效而便捷的方案。亲电子的二氯氰基甲基自由基是通过铜催化的单电子转移(SET)过程从容易获得的三氯乙腈中生成的,经过自由基加成后与N芳基丙烯酰胺的缺电子C = C键结合,然后进行分子内环化,从而提供二氯氰基甲基官能化的吲哚。在该转化中不需要外部氧化剂。
更新日期:2020-11-19
中文翻译:

铜(I)催化生成的二氯氰基甲基自由基引发的级联反应合成二氯氰基甲基官能化的吲哚
据报道,通过二氯氰基甲基自由基与N-芳基丙烯酰胺的级联反应,合成二氯氰基甲基官能化的吲哚的有效而便捷的方案。亲电子的二氯氰基甲基自由基是通过铜催化的单电子转移(SET)过程从容易获得的三氯乙腈中生成的,经过自由基加成后与N芳基丙烯酰胺的缺电子C = C键结合,然后进行分子内环化,从而提供二氯氰基甲基官能化的吲哚。在该转化中不需要外部氧化剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号