当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
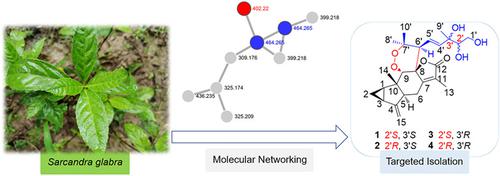
Sarcaglarols A—D, Lindenane−Monoterpene Heterodimers from Sarcandra glabra Based on Molecular Networks
Chinese Journal of Chemistry ( IF 5.4 ) Pub Date : 2020-09-11 , DOI: 10.1002/cjoc.202000456 Yongyue Wang 1 , Zhirong Cui 1 , Jun Chi 1 , Pengfei Tang 1 , Meihui Zhang 1 , Jixin Li 1 , Yongyi Li 1 , Hao Zhang 1 , Jun Luo 1 , Lingyi Kong 1
Chinese Journal of Chemistry ( IF 5.4 ) Pub Date : 2020-09-11 , DOI: 10.1002/cjoc.202000456 Yongyue Wang 1 , Zhirong Cui 1 , Jun Chi 1 , Pengfei Tang 1 , Meihui Zhang 1 , Jixin Li 1 , Yongyi Li 1 , Hao Zhang 1 , Jun Luo 1 , Lingyi Kong 1
Affiliation
|
Sarcaglarols A—D (1—4), two pairs of lindenane−monoterpene heterodimers fused by a 1,2‐dioxane moiety, were discovered and isolated from the leaves of Sarcandra glabra guided by MS/MS molecular networking‐based strategy. Their planar structures, absolute configurations of basic skeleton and flexible polyhydric side chain were established by analysis of HRESIMS, NMR spectroscopic data, ECD spectrum, and the X‐ray diffraction study of isopropylidene derivatives. An intermolecular [2+2+2] cycloaddition may play a key role in the biosynthesis pathway of the 1,2‐dioxane moiety fused lindenane−monoterpene heterodimer skeleton, which can be recognized as the biogenetic precursors of our previous reported lindenane−normonoterpene conjugates. In addition, compounds 1, 3 and 4 exhibited moderate inhibitory effects of lipid accumulation in free fatty acid‐exposed L02 cells.
中文翻译:

基于分子网络的Sarcandra glabra Sarcaglarols A-D,Lindenane-Monoterpene异二聚体
Sarcaglarols A-d(1 - 4)中,两对由1,2-二恶烷基部分稠合lindenane-单萜的异二聚体的,被发现并从叶子中分离草珊瑚通过MS / MS基于分子联网策略引导。通过分析HRESIMS,NMR光谱数据,ECD光谱和异亚丙基衍生物的X射线衍射研究,确定了它们的平面结构,基本骨架的绝对构型和柔性多元侧链。分子间的[2 + 2 + 2]环加成反应可能在1,2-二恶烷部分稠合的林丹-单萜异二聚体骨架的生物合成途径中发挥关键作用,这可以被认为是我们先前报道的林丹-北单萜共轭物的生物遗传前体。 。此外,化合物1,3和4的脂质积累的游离脂肪酸暴露L02细胞表现出中度抑制效果。
更新日期:2020-09-11
中文翻译:

基于分子网络的Sarcandra glabra Sarcaglarols A-D,Lindenane-Monoterpene异二聚体
Sarcaglarols A-d(1 - 4)中,两对由1,2-二恶烷基部分稠合lindenane-单萜的异二聚体的,被发现并从叶子中分离草珊瑚通过MS / MS基于分子联网策略引导。通过分析HRESIMS,NMR光谱数据,ECD光谱和异亚丙基衍生物的X射线衍射研究,确定了它们的平面结构,基本骨架的绝对构型和柔性多元侧链。分子间的[2 + 2 + 2]环加成反应可能在1,2-二恶烷部分稠合的林丹-单萜异二聚体骨架的生物合成途径中发挥关键作用,这可以被认为是我们先前报道的林丹-北单萜共轭物的生物遗传前体。 。此外,化合物1,3和4的脂质积累的游离脂肪酸暴露L02细胞表现出中度抑制效果。

























 京公网安备 11010802027423号
京公网安备 11010802027423号