Tetrahedron Letters ( IF 1.8 ) Pub Date : 2020-09-11 , DOI: 10.1016/j.tetlet.2020.152447 Ngiap-Kie Lim , Haiming Zhang , C. Gregory Sowell , Francis Gosselin
|
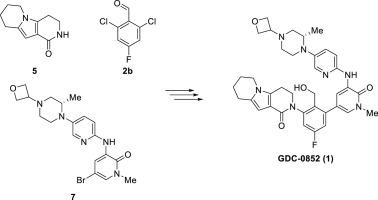
The development of an expedient synthesis to GDC-0852 (1), a reversible BTK inhibitor drug candidate, is described. The key starting material tricyclic lactam 5 was prepared by an annulation reaction of unprotected piperidine-2-carbaldehyde HCl salt (20) and N-Boc piperidine-2,4-dione 21 in a safe and scalable manner. A highly selective Pd-catalyzed C N coupling of lactam 5 and linker 2a, followed by Suzuki−Miyaura coupling to fragment 8 subsequently provided a direct and convergent access to the penultimate 17. A simple NaBH4 aldehyde reduction completed the synthesis to GDC-0852 (1) in high yield (54% over 3 steps from 5) and purity (99.0 A% HPLC).
N coupling of lactam 5 and linker 2a, followed by Suzuki−Miyaura coupling to fragment 8 subsequently provided a direct and convergent access to the penultimate 17. A simple NaBH4 aldehyde reduction completed the synthesis to GDC-0852 (1) in high yield (54% over 3 steps from 5) and purity (99.0 A% HPLC).
中文翻译:

适用于目的合成布鲁顿酪氨酸激酶抑制剂GDC-0852
描述了向可逆的BTK抑制剂候选药物GDC-0852(1)的合成方法的开发。通过未保护的哌啶-2-甲醛甲醛盐酸盐(20)与N -Boc哌啶-2,4-二酮21的环合反应,以安全且可扩展的方式制备关键起始原料三环内酰胺5。内酰胺5和接头2a的高选择性Pd催化的C N偶联,然后是Suzuki-Miyaura偶联至片段8,随后直接并会聚地进入倒数第二个17。一个简单的NaBH 4 乙醛的还原以高收率(从5开始的3个步骤为54%)和纯度(99.0A%HPLC)完成了向GDC-0852(1)的合成。
乙醛的还原以高收率(从5开始的3个步骤为54%)和纯度(99.0A%HPLC)完成了向GDC-0852(1)的合成。

























 京公网安备 11010802027423号
京公网安备 11010802027423号