Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy ( IF 4.4 ) Pub Date : 2020-09-11 , DOI: 10.1016/j.saa.2020.118937 Sha Ding , Aixiang Xu , Aokui Sun , Yong Xia , Yuejun Liu
|
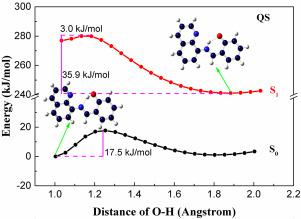
The effects of substituent on excited-state intramolecular proton transfer (ESIPT) and hydrogen bonding of N-(8-Quinolyl) salicylaldimine (QS) have been studied by theoretical calculation with DFT and TDDFT. The representative electron-withdrawing nitryl and electron-donating methoxyl were selected to analyze the effects on geometries, intramolecular hydrogen bond interaction, absorption/fluorescence spectra, and the ESIPT process. The configurations of the three molecules (QS, QS-OMe and QS-NO2) were optimized in the ground and excited states. The structure parameters, infrared spectra, hydrogen bond interactions, frontier molecular orbitals, absorption/fluorescence spectra, and potential curves have cross-validated the current results. The results show that the introduction of substituent results in a bathochromic-shift of the absorption and fluorescence spectra with large Stokes shift, and is more beneficial to the ESIPT process. The current work will be beneficial to the improvement of ESIPT properties and deepen understanding of the mechanism of ESIPT process.
中文翻译:

取代基对ESIPT的影响和N-(8-喹啉)水杨醛亚胺的氢键机理:详细的理论探索
通过DFT和TDDFT的理论计算,研究了取代基对N-(8-喹啉基)水杨醛亚胺(QS)的激发态分子内质子转移(ESIPT)和氢键的影响。选择代表性的吸电子的腈基和给电子的甲氧基来分析对几何形状,分子内氢键相互作用,吸收/荧光光谱和ESIPT过程的影响。QS,QS-OMe和QS-NO 2这三个分子的构型)在基态和激发态下进行了优化。结构参数,红外光谱,氢键相互作用,前沿分子轨道,吸收/荧光光谱以及电势曲线对当前结果进行了交叉验证。结果表明,取代基的引入导致吸收和荧光光谱发生红移,斯托克斯位移较大,对ESIPT工艺更有利。当前的工作将有利于提高ESIPT的性能,加深对ESIPT过程机理的理解。


























 京公网安备 11010802027423号
京公网安备 11010802027423号