Frontiers of Chemical Science and Engineering ( IF 4.5 ) Pub Date : 2020-09-11 , DOI: 10.1007/s11705-020-1957-2 Kai Cai , Ying Li , Hongbao Shen , Zaizhe Cheng , Shouying Huang , Yue Wang , Xinbin Ma
|
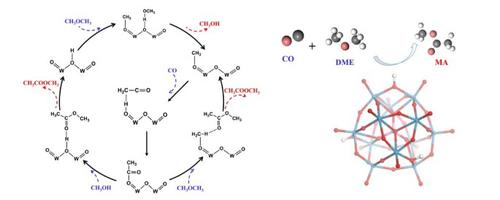
Dimethyl ether (DME) carbonylation is considered as a key step for a promising route to produce ethanol from syngas. Heteropolyacids (HPAs) are proved to be efficient catalysts for DME carbonylation. In this work, the reaction mechanism of DME carbonylation was studied theoretically by using density functional theory calculations on two typical HPA models (HPW, HSiW). The whole process consists of three stages: DME dissociative adsorption, insertion of CO into methoxyl group and formation of product methyl acetate. The activation barriers of all possible elementary steps, especially two possible paths for CO insertion were calculated to obtain the most favorable reaction mechanism and rate-limiting step. Furthermore, the effect of the acid strength of Brønsted acid sites on reactivity was studied by comparing the activation barriers over HPW and HSiW with different acid strength, which was determined by calculating the deprotonation energy, Mulliken population analyses and adsorption energies of pyridine.
中文翻译:

杂多酸催化剂上二甲醚羰基化反应机理的密度泛函理论研究
二甲醚(DME)羰基化被认为是从合成气生产乙醇的有前途的关键步骤。杂多酸(HPA)被证明是DME羰基化的有效催化剂。在这项工作中,通过在两个典型的HPA模型(HPW,HSiW)上使用密度泛函理论计算,对DME羰基化的反应机理进行了理论研究。整个过程包括三个阶段:DME的离解吸附,将CO插入甲氧基和形成乙酸甲酯。计算了所有可能的基本步骤,特别是CO插入的两个可能路径的活化势垒,以获得最有利的反应机理和限速步骤。此外,



























 京公网安备 11010802027423号
京公网安备 11010802027423号