当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
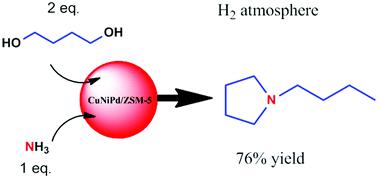
One-pot synthesis of 1-butylpyrrolidine and its derivatives from aqueous ammonia and 1,4-butandiol over CuNiPd/ZSM-5 catalysts
New Journal of Chemistry ( IF 3.3 ) Pub Date : 2020-09-10 , DOI: 10.1039/d0nj02224a Yan Long 1, 2, 3, 4, 5 , Shimin Liu 1, 2, 3, 4, 5 , Xiangyuan Ma 1, 2, 3, 4, 5 , Liujin Lu 1, 2, 3, 4, 5 , Yude He 1, 2, 3, 4, 5 , Youquan Deng 1, 2, 3, 4, 5
New Journal of Chemistry ( IF 3.3 ) Pub Date : 2020-09-10 , DOI: 10.1039/d0nj02224a Yan Long 1, 2, 3, 4, 5 , Shimin Liu 1, 2, 3, 4, 5 , Xiangyuan Ma 1, 2, 3, 4, 5 , Liujin Lu 1, 2, 3, 4, 5 , Yude He 1, 2, 3, 4, 5 , Youquan Deng 1, 2, 3, 4, 5
Affiliation
|
The synthesis of 1-butylpyrrolidine and its derivatives (1-butylpyrrolidine with a little of 1-butenylpyrrolidines) was developed via a one-pot method from ammonia and 1,4-butandiol. Here, the product of 1-butylpyrrolidine was emphatically investigated, and the yield was 76% under the optimized conditions. Such a route was realized through successive N-alkylation using aqueous ammonia as the nitrogen source over the CuNiPd/ZSM-5 catalyst, which was prepared by a simple incipient wetness method. In this route, 1,4-butandiol not only participated in the formation of the N-heterocycle, but also acted as an alkylating reagent. This work offers a straightforward, economical route for 1-butylpyrrolidine and its derivatives.
中文翻译:

在CuNiPd / ZSM-5催化剂上由氨水和1,4-丁二醇一锅法合成1-丁基吡咯烷及其衍生物
通过一锅法从氨和1,4-丁二醇开发了1-丁基吡咯烷及其衍生物(1-丁基吡咯烷和少量1-丁烯基吡咯烷)的合成方法。在此,着重研究了1-丁基吡咯烷的产物,在最佳条件下产率为76%。通过使用氨水作为氮源在CuNiPd / ZSM-5催化剂上连续N-烷基化来实现这种途径,该CuNiPd / ZSM-5催化剂是通过简单的初湿法制备的。在这种途径中,1,4-丁二醇不仅参与了N-杂环的形成,而且还充当了烷基化试剂。这项工作为1-丁基吡咯烷及其衍生物提供了一种简单,经济的途径。
更新日期:2020-10-15
中文翻译:

在CuNiPd / ZSM-5催化剂上由氨水和1,4-丁二醇一锅法合成1-丁基吡咯烷及其衍生物
通过一锅法从氨和1,4-丁二醇开发了1-丁基吡咯烷及其衍生物(1-丁基吡咯烷和少量1-丁烯基吡咯烷)的合成方法。在此,着重研究了1-丁基吡咯烷的产物,在最佳条件下产率为76%。通过使用氨水作为氮源在CuNiPd / ZSM-5催化剂上连续N-烷基化来实现这种途径,该CuNiPd / ZSM-5催化剂是通过简单的初湿法制备的。在这种途径中,1,4-丁二醇不仅参与了N-杂环的形成,而且还充当了烷基化试剂。这项工作为1-丁基吡咯烷及其衍生物提供了一种简单,经济的途径。


























 京公网安备 11010802027423号
京公网安备 11010802027423号