当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
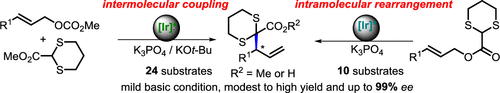
Enantioselective α-Functionalization of 1,3-Dithianes by Iridium-Catalyzed Allylic Substitution.
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-09-10 , DOI: 10.1021/acs.joc.0c01683 Panpan Wang 1 , Qian Jiang 1 , Ruibo Zhao 1 , Xingang Xie 1 , Shouchu Tang 1, 2 , Xiaolei Wang 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-09-10 , DOI: 10.1021/acs.joc.0c01683 Panpan Wang 1 , Qian Jiang 1 , Ruibo Zhao 1 , Xingang Xie 1 , Shouchu Tang 1, 2 , Xiaolei Wang 1
Affiliation
|
An iridium-catalyzed asymmetric allylic substitution reaction with 2-alkoxy carbonyl-1,3-dithianes has been achieved with high regio- and enantioselectivities. The transformation provides a new method for the enantioselective α-functionalization of dithianes. The corresponding dithiane-containing products are easily converted into many other derivatives with high yields and enantioselectivities.
中文翻译:

铱催化的烯丙基取代对1,3-二硫醚的对映选择性α-官能化。
用2-区域烷氧基羰基-1,3-二硫杂环丁烷进行铱催化的不对称烯丙基取代反应,具有很高的区域选择性和对映选择性。该转化为二噻烷的对映选择性α-官能化提供了一种新方法。相应的含二噻吩的产物易于转化成许多其他具有高产率和对映选择性的衍生物。
更新日期:2020-10-02
中文翻译:

铱催化的烯丙基取代对1,3-二硫醚的对映选择性α-官能化。
用2-区域烷氧基羰基-1,3-二硫杂环丁烷进行铱催化的不对称烯丙基取代反应,具有很高的区域选择性和对映选择性。该转化为二噻烷的对映选择性α-官能化提供了一种新方法。相应的含二噻吩的产物易于转化成许多其他具有高产率和对映选择性的衍生物。


























 京公网安备 11010802027423号
京公网安备 11010802027423号