当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Divergent Synthesis of Isonitriles and Nitriles by Palladium-Catalyzed Benzylic Substitution with TMSCN.
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-09-09 , DOI: 10.1021/acs.joc.0c01861 Kento Asai 1 , Koji Hirano 1 , Masahiro Miura 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-09-09 , DOI: 10.1021/acs.joc.0c01861 Kento Asai 1 , Koji Hirano 1 , Masahiro Miura 1
Affiliation
|
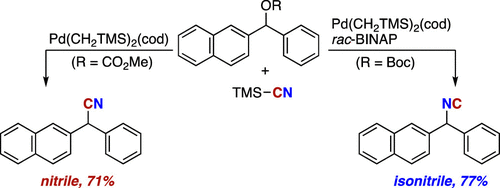
Ligand-controlled palladium-catalyzed divergent synthesis of isonitriles and nitriles from benzylic carbonates and TMSCN has been developed. The BINAP- or DPEphos-ligated palladium catalyst selectively provides the corresponding benzylic isonitriles, whereas their regioisomers, benzylic nitriles, are formed exclusively under phosphine ligand-free conditions. Mechanistic studies reveal that isonitrile is the primary product under both conditions, but it is isomerized into nitrile in the absence of ancillary phosphine ligands.
中文翻译:

钯催化苯甲酰胺与TMSCN取代合成异腈和腈
已经开发了由碳酸苄基酯和TMSCN进行配体控制的钯催化的异腈和腈的发散合成。BINAP或DPEphos连接的钯催化剂选择性地提供相应的苄基异腈,而它们的区域异构体苄基腈仅在无膦配体的条件下形成。机理研究表明,在两种条件下,异腈都是主要产物,但在没有辅助膦配体的情况下,它会异构化为腈。
更新日期:2020-10-02
中文翻译:

钯催化苯甲酰胺与TMSCN取代合成异腈和腈
已经开发了由碳酸苄基酯和TMSCN进行配体控制的钯催化的异腈和腈的发散合成。BINAP或DPEphos连接的钯催化剂选择性地提供相应的苄基异腈,而它们的区域异构体苄基腈仅在无膦配体的条件下形成。机理研究表明,在两种条件下,异腈都是主要产物,但在没有辅助膦配体的情况下,它会异构化为腈。



























 京公网安备 11010802027423号
京公网安备 11010802027423号