当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Conformational Lock of Glycosyl Donors Using Cyclic Carbamates
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-09-10 , DOI: 10.1002/ejoc.202001130 Jaime Moyano Villameriel 1 , Christian Marcus Pedersen 1
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-09-10 , DOI: 10.1002/ejoc.202001130 Jaime Moyano Villameriel 1 , Christian Marcus Pedersen 1
Affiliation
|
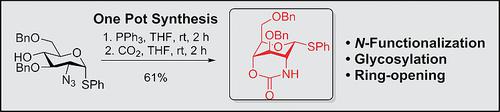
A simple synthesis of a 2,4‐tethered glucosaminyl donor has been developed. The commonly synthesized 2‐azido‐2‐deoxy glucosyl donor is transferred into the cyclic carbamate using a Staudinger azide reduction followed by CO2 capture giving an isocyate. This highly reactive intermediate readily participate in an intramolecular ring‐closure with the 4‐OH in a one‐pot fashion. The novel donor type achieved can then be N‐functionalized and used in glycosylation reactions.

中文翻译:

使用环状氨基甲酸酯的糖基供体的构象锁定
已开发出2,4-束缚的氨基葡萄糖氨基供体的简单合成方法。使用Staudinger叠氮化物还原法将通常合成的2-叠氮基-2-脱氧葡萄糖基供体转移到环状氨基甲酸酯中,然后捕获CO 2以获得异氰酸酯。这种高反应性中间体很容易以一锅方式与4-OH参与分子内闭环。然后可以将N型官能化的新型供体用于糖基化反应。

更新日期:2020-11-02

中文翻译:

使用环状氨基甲酸酯的糖基供体的构象锁定
已开发出2,4-束缚的氨基葡萄糖氨基供体的简单合成方法。使用Staudinger叠氮化物还原法将通常合成的2-叠氮基-2-脱氧葡萄糖基供体转移到环状氨基甲酸酯中,然后捕获CO 2以获得异氰酸酯。这种高反应性中间体很容易以一锅方式与4-OH参与分子内闭环。然后可以将N型官能化的新型供体用于糖基化反应。



























 京公网安备 11010802027423号
京公网安备 11010802027423号