当前位置:
X-MOL 学术
›
Biotechnol. Bioeng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Displacement to separate host-cell proteins and aggregates in cation-exchange chromatography of monoclonal antibodies.
Biotechnology and Bioengineering ( IF 3.8 ) Pub Date : 2020-09-10 , DOI: 10.1002/bit.27559 Ohnmar Khanal 1 , Vijesh Kumar 1 , Abraham M Lenhoff 1
Biotechnology and Bioengineering ( IF 3.8 ) Pub Date : 2020-09-10 , DOI: 10.1002/bit.27559 Ohnmar Khanal 1 , Vijesh Kumar 1 , Abraham M Lenhoff 1
Affiliation
|
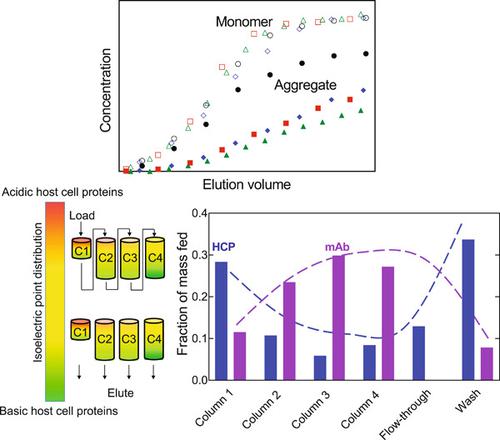
An efficient and consistent method of monoclonal antibody (mAb) purification can improve process productivity and product consistency. Although protein A chromatography removes most host‐cell proteins (HCPs), mAb aggregates and the remaining HCPs are challenging to remove in a typical bind‐and‐elute cation‐exchange chromatography (CEX) polishing step. A variant of the bind‐and‐elute mode is the displacement mode, which allows strongly binding impurities to be preferentially retained and significantly improves resin utilization. Improved resin utilization renders displacement chromatography particularly suitable in continuous chromatography operations. In this study we demonstrate and exploit sample displacement between a mAb and impurities present at low prevalence (0.002%–1.4%) using different multicolumn designs and recycling. Aggregate displacement depends on the residence time, sample concentration, and solution environment, the latter by enhancing the differences between the binding affinities of the product and the impurities. Displacement among the mAb and low‐prevalence HCPs resulted in an effectively bimodal‐like distribution of HCPs along the length of a multi‐column system, with the mAb separating the relatively more basic group of HCPs from those that are more acidic. Our findings demonstrate that displacement of low‐prevalence impurities along multiple CEX columns allows for selective separation of mAb aggregates and HCPs that persist through protein A chromatography.
中文翻译:

在单克隆抗体的阳离子交换层析中置换以分离宿主细胞蛋白质和聚集体。
一种高效且一致的单克隆抗体 (mAb) 纯化方法可以提高过程生产率和产品一致性。尽管蛋白 A 层析去除了大多数宿主细胞蛋白 (HCP),但在典型的结合和洗脱阳离子交换层析 (CEX) 精制步骤中,要去除 mAb 聚集体和剩余的 HCP 具有挑战性。结合和洗脱模式的一个变体是置换模式,它允许优先保留强结合的杂质并显着提高树脂利用率。改进的树脂利用率使得置换色谱特别适用于连续色谱操作。在本研究中,我们使用不同的多柱设计和回收利用证明并利用 mAb 和低流行率 (0.002%–1.4%) 杂质之间的样品置换。聚集体置换取决于停留时间、样品浓度和溶液环境,后者通过增强产物与杂质的结合亲和力之间的差异来实现。mAb 和低流行 HCP 之间的置换导致 HCP 沿着多柱系统的长度呈双峰状分布,mAb 将相对碱性的 HCP 组与酸性更强的 HCP 分开。我们的研究结果表明,沿多个 CEX 柱置换低流行性杂质可以选择性分离 mAb 聚集体和通过蛋白 A 色谱持续存在的 HCP。mAb 和低流行 HCP 之间的置换导致 HCP 沿着多柱系统的长度呈双峰状分布,mAb 将相对碱性的 HCP 组与酸性更强的 HCP 分开。我们的研究结果表明,沿多个 CEX 柱置换低流行性杂质可以选择性分离 mAb 聚集体和通过蛋白 A 色谱持续存在的 HCP。mAb 和低流行 HCP 之间的置换导致 HCP 沿着多柱系统的长度呈双峰状分布,mAb 将相对碱性的 HCP 组与酸性更强的 HCP 分开。我们的研究结果表明,沿多个 CEX 柱置换低流行性杂质可以选择性分离 mAb 聚集体和通过蛋白 A 色谱持续存在的 HCP。
更新日期:2020-09-10
中文翻译:

在单克隆抗体的阳离子交换层析中置换以分离宿主细胞蛋白质和聚集体。
一种高效且一致的单克隆抗体 (mAb) 纯化方法可以提高过程生产率和产品一致性。尽管蛋白 A 层析去除了大多数宿主细胞蛋白 (HCP),但在典型的结合和洗脱阳离子交换层析 (CEX) 精制步骤中,要去除 mAb 聚集体和剩余的 HCP 具有挑战性。结合和洗脱模式的一个变体是置换模式,它允许优先保留强结合的杂质并显着提高树脂利用率。改进的树脂利用率使得置换色谱特别适用于连续色谱操作。在本研究中,我们使用不同的多柱设计和回收利用证明并利用 mAb 和低流行率 (0.002%–1.4%) 杂质之间的样品置换。聚集体置换取决于停留时间、样品浓度和溶液环境,后者通过增强产物与杂质的结合亲和力之间的差异来实现。mAb 和低流行 HCP 之间的置换导致 HCP 沿着多柱系统的长度呈双峰状分布,mAb 将相对碱性的 HCP 组与酸性更强的 HCP 分开。我们的研究结果表明,沿多个 CEX 柱置换低流行性杂质可以选择性分离 mAb 聚集体和通过蛋白 A 色谱持续存在的 HCP。mAb 和低流行 HCP 之间的置换导致 HCP 沿着多柱系统的长度呈双峰状分布,mAb 将相对碱性的 HCP 组与酸性更强的 HCP 分开。我们的研究结果表明,沿多个 CEX 柱置换低流行性杂质可以选择性分离 mAb 聚集体和通过蛋白 A 色谱持续存在的 HCP。mAb 和低流行 HCP 之间的置换导致 HCP 沿着多柱系统的长度呈双峰状分布,mAb 将相对碱性的 HCP 组与酸性更强的 HCP 分开。我们的研究结果表明,沿多个 CEX 柱置换低流行性杂质可以选择性分离 mAb 聚集体和通过蛋白 A 色谱持续存在的 HCP。


























 京公网安备 11010802027423号
京公网安备 11010802027423号