Cell ( IF 64.5 ) Pub Date : 2020-09-10 , DOI: 10.1016/j.cell.2020.07.039 François Bonnay 1 , Ana Veloso 2 , Victoria Steinmann 1 , Thomas Köcher 3 , Merve Deniz Abdusselamoglu 1 , Sunanjay Bajaj 1 , Elisa Rivelles 4 , Lisa Landskron 1 , Harald Esterbauer 4 , Robert P Zinzen 2 , Juergen A Knoblich 1
|
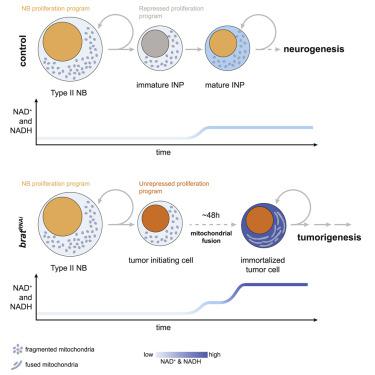
Metabolic reprogramming is a key feature of many cancers, but how and when it contributes to tumorigenesis remains unclear. Here we demonstrate that metabolic reprogramming induced by mitochondrial fusion can be rate-limiting for immortalization of tumor-initiating cells (TICs) and trigger their irreversible dedication to tumorigenesis. Using single-cell transcriptomics, we find that Drosophila brain tumors contain a rapidly dividing stem cell population defined by upregulation of oxidative phosphorylation (OxPhos). We combine targeted metabolomics and in vivo genetic screening to demonstrate that OxPhos is required for tumor cell immortalization but dispensable in neural stem cells (NSCs) giving rise to tumors. Employing an in vivo NADH/NAD+ sensor, we show that NSCs precisely increase OxPhos during immortalization. Blocking OxPhos or mitochondrial fusion stalls TICs in quiescence and prevents tumorigenesis through impaired NAD+ regeneration. Our work establishes a unique connection between cellular metabolism and immortalization of tumor-initiating cells.
中文翻译:

氧化代谢在肿瘤发生过程中驱动神经干细胞永生化。
代谢重编程是许多癌症的关键特征,但尚不清楚其如何以及何时促进肿瘤发生。在这里,我们证明了由线粒体融合诱导的代谢重编程可以限制肿瘤引发细胞(TICs)的永生化,并触发它们对肿瘤发生的不可逆转的奉献。使用单细胞转录组学,我们发现果蝇脑肿瘤包含一个快速分裂的干细胞群体,该群体由氧化磷酸化(OxPhos)的上调定义。我们结合了靶向代谢组学和体内遗传筛选,以证明OxPhos是永生化肿瘤细胞所必需的,但在神经干细胞(NSCs)中却是不可缺少的,从而引起肿瘤。使用体内NADH / NAD +传感器,我们显示NSC可以在永生化过程中精确地增加OxPhos。阻断OxPhos或线粒体融合可阻止TIC处于静止状态,并通过受损的NAD +再生阻止肿瘤发生。我们的工作在细胞代谢和肿瘤引发细胞永生化之间建立了独特的联系。



























 京公网安备 11010802027423号
京公网安备 11010802027423号