Cancer Cell ( IF 50.3 ) Pub Date : 2020-09-10 , DOI: 10.1016/j.ccell.2020.08.007 Padmanee Sharma 1 , Russell K Pachynski 2 , Vivek Narayan 3 , Aude Fléchon 4 , Gwenaelle Gravis 5 , Matthew D Galsky 6 , Hakim Mahammedi 7 , Akash Patnaik 8 , Sumit K Subudhi 1 , Marika Ciprotti 9 , Burcin Simsek 10 , Abdel Saci 10 , Yanhua Hu 10 , G Celine Han 10 , Karim Fizazi 11
|
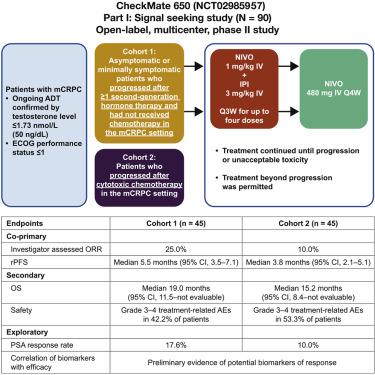
Metastatic castration-resistant prostate cancer (mCRPC) is immunologically “cold” and predominantly resistant to immune checkpoint therapy due to few tumor-infiltrating T cells. Ipilimumab (anti-CTLA-4) or anti-PD-1/PD-L1 monotherapy failed to show a significant benefit. Although the PD-1/PD-L1 pathway is minimally expressed in prostate tumors, we previously demonstrated that PD-1/PD-L1 expression increases as a compensatory inhibitory pathway in parallel with an ipilimumab-induced increase in tumor-infiltrating T cells. Here, we report the largest trial to date in mCRPC with anti-CTLA-4 plus anti-PD-1 (nivolumab 1 mg/kg plus ipilimumab 3 mg/kg; CheckMate 650, NCT02985957). With median follow-ups of 11.9 and 13.5 months in cohorts 1 (pre-chemotherapy; n = 45) and 2 (post-chemotherapy; n = 45), objective response rate was 25% and 10%, and median overall survival was 19.0 and 15.2 months, respectively. Four patients, two in each cohort, had complete responses. Exploratory studies identify potential biomarkers of response. Grade 3–4 treatment-related adverse events have occurred in ∼42%–53% of patients, with four treatment-related deaths. Therefore, dose/schedule modifications have been implemented.
中文翻译:

Nivolumab加伊匹木单抗用于转移性去势抵抗性前列腺癌:CheckMate 650试验中患者的初步分析。
转移性去势抵抗性前列腺癌(mCRPC)在免疫学上是“冷”的,并且由于很少有肿瘤浸润性T细胞而对免疫检查点疗法具有主要抵抗力。伊匹木单抗(抗CTLA-4)或抗PD-1 / PD-L1单一疗法未能显示出明显的获益。尽管PD-1 / PD-L1途径在前列腺肿瘤中的表达最少,但我们先前证明PD-1 / PD-L1的表达作为补偿性抑制途径的增加与伊立木单抗诱导的肿瘤浸润T细胞的增加平行。在这里,我们报道了迄今为止在mCRPC中使用抗CTLA-4和抗PD-1(诺华单抗1 mg / kg加伊匹单抗3 mg / kg; CheckMate 650,NCT02985957)进行的最大试验。队列1(化疗前; n = 45)和队列2(化疗后; n = 45)的中位随访时间分别为11.9和13.5个月,客观缓解率为25%和10%,中位总生存期分别为19.0和15.2个月。四名患者,每组中两名,完全缓解。探索性研究确定了潜在的反应生物标志物。约有42%–53%的患者发生了3–4级与治疗相关的不良事件,其中有4名与治疗相关的死亡。因此,已经实现了剂量/时间表的修改。



























 京公网安备 11010802027423号
京公网安备 11010802027423号