当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
One-Pot Condensation of a Bicyclo[1.1.1]pentasilane through Elimination of Iodotrimethylsilane Assisted by a Lewis Base
Organometallics ( IF 2.8 ) Pub Date : 2020-09-09 , DOI: 10.1021/acs.organomet.0c00514 Yuki Yokouchi 1 , Takeaki Iwamoto 1
Organometallics ( IF 2.8 ) Pub Date : 2020-09-09 , DOI: 10.1021/acs.organomet.0c00514 Yuki Yokouchi 1 , Takeaki Iwamoto 1
Affiliation
|
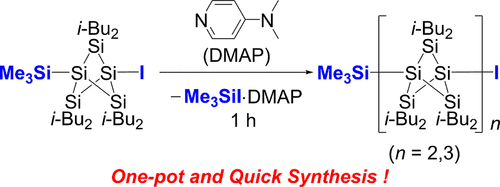
Development of efficient bond formation reactions remains important in organic and main-group-element chemistry. Herein, we report the metal-free one-pot condensation reaction of 1-iodo-3-trimethylsilyl-substituted bicyclo[1.1.1]pentasilane (BPS) in the presence of 4-(dimethylamino)pyridine (DMAP) providing oligomers of BPS, persila[n]staffanes (n = 2, 3), which involves the successive Si–Si bond formation reaction accompanied by the concomitant formation of iodotrimethylsilane (TMSI) in the form of a DMAP complex. The computational study suggests that the formation of a pentasila[1.1.1]propellane–DMAP complex resulting from the elimination of TMSI from the BPS is a key intermediate.
中文翻译:

通过路易斯碱辅助的碘代三甲基硅烷的一锅缩合双环[1.1.1]戊硅烷
有效的键形成反应的发展在有机和主族元素化学中仍然很重要。在此,我们报告了在4-(二甲基氨基)吡啶(DMAP)存在下,由1-碘-3-三甲基甲硅烷基取代的双环[1.1.1]五硅烷(BPS)进行的无金属一锅缩合反应,Persila [ n ]硫烷(n = 2,3),涉及到连续的Si-Si键形成反应,并伴随以DMAP络合物的形式形成碘三甲基硅烷(TMSI)。计算研究表明,从BPS中消除TMSI导致的戊戊[1.1.1]丙炔-DMAP复合物的形成是关键的中间产物。
更新日期:2020-09-28
中文翻译:

通过路易斯碱辅助的碘代三甲基硅烷的一锅缩合双环[1.1.1]戊硅烷
有效的键形成反应的发展在有机和主族元素化学中仍然很重要。在此,我们报告了在4-(二甲基氨基)吡啶(DMAP)存在下,由1-碘-3-三甲基甲硅烷基取代的双环[1.1.1]五硅烷(BPS)进行的无金属一锅缩合反应,Persila [ n ]硫烷(n = 2,3),涉及到连续的Si-Si键形成反应,并伴随以DMAP络合物的形式形成碘三甲基硅烷(TMSI)。计算研究表明,从BPS中消除TMSI导致的戊戊[1.1.1]丙炔-DMAP复合物的形成是关键的中间产物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号