当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
BF3·OEt2-TFAA Mediated Tetra-Functionalization of Amino Acids - Synthesis of Di- and Tri-Substituted 2-Trifluoromethyl Oxazoles in One Pot.
Organic Letters ( IF 5.2 ) Pub Date : 2020-09-09 , DOI: 10.1021/acs.orglett.0c02484 Velusamy Karuppusamy 1, 2 , Andivelu Ilangovan 1
Organic Letters ( IF 5.2 ) Pub Date : 2020-09-09 , DOI: 10.1021/acs.orglett.0c02484 Velusamy Karuppusamy 1, 2 , Andivelu Ilangovan 1
Affiliation
|
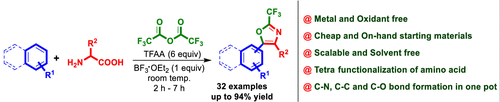
A highly efficient, TFAA-BF3·OEt2 mediated multicomponent coupling of amino acid, TFAA, and aromatics provides a broad library of 2-trifluoromethyl equipped 2,5-disubstituted/2,4,5-trisubstituted oxazoles or N-(trifluoroacetyl)-β-aminoalkyl ketones. This amino acid tetra-functionalization approach involves amidation (C–N), anhydride (C–O), Friedel–Crafts acylation (C–C), and Robinson–Gabriel annulation (C–O) followed by dehydrative aromatization. This reaction takes place under operationally simple, mild, and metal-free conditions using readily available amino acids and aromatic compounds.
中文翻译:

BF3·OEt2-TFAA介导的氨基酸四官能化-一锅合成二和三取代的2-三氟甲基恶唑。
氨基酸,TFAA和芳香族化合物的高效,TFAA-BF 3 ·OEt 2介导的多组分偶联提供了配备2-三氟甲基的2,5-二取代/ 2,4,5-三取代的恶唑或N-(三氟乙酰基)的宽泛文库)-β-氨基烷基酮。这种氨基酸四官能化方法包括酰胺化(C–N),酸酐(C–O),弗瑞德-克拉夫茨酰化(CC)和鲁宾逊-加布里埃尔环化(C–O),然后进行脱水芳构化。使用容易获得的氨基酸和芳族化合物,该反应在操作简单,温和且不含金属的条件下进行。
更新日期:2020-09-20
中文翻译:

BF3·OEt2-TFAA介导的氨基酸四官能化-一锅合成二和三取代的2-三氟甲基恶唑。
氨基酸,TFAA和芳香族化合物的高效,TFAA-BF 3 ·OEt 2介导的多组分偶联提供了配备2-三氟甲基的2,5-二取代/ 2,4,5-三取代的恶唑或N-(三氟乙酰基)的宽泛文库)-β-氨基烷基酮。这种氨基酸四官能化方法包括酰胺化(C–N),酸酐(C–O),弗瑞德-克拉夫茨酰化(CC)和鲁宾逊-加布里埃尔环化(C–O),然后进行脱水芳构化。使用容易获得的氨基酸和芳族化合物,该反应在操作简单,温和且不含金属的条件下进行。


























 京公网安备 11010802027423号
京公网安备 11010802027423号