当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Asymmetric Construction of Cyclobutanes via Direct Vinylogous Michael Addition/Cyclization of β,γ-Unsaturated Amides.
Organic Letters ( IF 5.2 ) Pub Date : 2020-09-09 , DOI: 10.1021/acs.orglett.0c02488 Yuzhen Chen 1 , Yichen Wang 1 , Shuzhong Wang 1 , Yan-Yan Ma 2 , Deng-Gao Zhao 2 , Ruoting Zhan 1 , Huicai Huang 1
Organic Letters ( IF 5.2 ) Pub Date : 2020-09-09 , DOI: 10.1021/acs.orglett.0c02488 Yuzhen Chen 1 , Yichen Wang 1 , Shuzhong Wang 1 , Yan-Yan Ma 2 , Deng-Gao Zhao 2 , Ruoting Zhan 1 , Huicai Huang 1
Affiliation
|
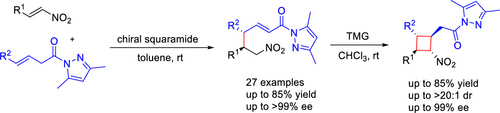
The construction of cyclobutanes has attracted much attention because of its unique four-membered ring skeleton. Herein, we report the highly enantioselective direct vinylogous Michael reaction of β,γ-unsaturated pyrazole amides and nitroolefin using a squaramide catalyst. Cyclobutane derivatives were obtained by subsequent cyclization in good yields (up to 85%) with excellent enantioselectivities (up to 99% ee). Importantly, the large-scale reaction experiment confirmed the reliability of the vinylogous reaction. Furthermore, the synthetic utility of the vinylogous adducts and cyclobutane derivatives has been realized.
中文翻译:

β,γ-不饱和酰胺的直接直链迈克尔加成/环化形成环戊二烯的不对称结构。
环丁烷的构建因其独特的四元环骨架而备受关注。在此,我们报道了使用方酰胺催化剂的β,γ-不饱和吡唑酰胺和硝基烯烃的高度对映选择性的直接乙烯基迈克尔反应。环丁烷衍生物是通过随后的环化反应以良好的收率(高达99%ee)以良好的收率(高达85%)获得的。重要的是,大规模反应实验证实了乙烯基反应的可靠性。此外,已经实现了乙烯基类加合物和环丁烷衍生物的合成用途。
更新日期:2020-09-20
中文翻译:

β,γ-不饱和酰胺的直接直链迈克尔加成/环化形成环戊二烯的不对称结构。
环丁烷的构建因其独特的四元环骨架而备受关注。在此,我们报道了使用方酰胺催化剂的β,γ-不饱和吡唑酰胺和硝基烯烃的高度对映选择性的直接乙烯基迈克尔反应。环丁烷衍生物是通过随后的环化反应以良好的收率(高达99%ee)以良好的收率(高达85%)获得的。重要的是,大规模反应实验证实了乙烯基反应的可靠性。此外,已经实现了乙烯基类加合物和环丁烷衍生物的合成用途。

























 京公网安备 11010802027423号
京公网安备 11010802027423号