当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
REDUCTION OF 3-NITRO-1,2,4-TRIAZOL-5-ONE (NTO) BY THE HEMATITE-AQUEOUS Fe(II) REDOX COUPLE.
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2020-09-09 , DOI: 10.1021/acs.est.0c03872 Paula A Cárdenas-Hernández 1 , Katelyn A Anderson 1 , Jimmy Murillo-Gelvez 1 , Dominic M Di Toro 1 , Herbert E Allen 1 , Richard F Carbonaro 2, 3 , Pei C Chiu 1
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2020-09-09 , DOI: 10.1021/acs.est.0c03872 Paula A Cárdenas-Hernández 1 , Katelyn A Anderson 1 , Jimmy Murillo-Gelvez 1 , Dominic M Di Toro 1 , Herbert E Allen 1 , Richard F Carbonaro 2, 3 , Pei C Chiu 1
Affiliation
|
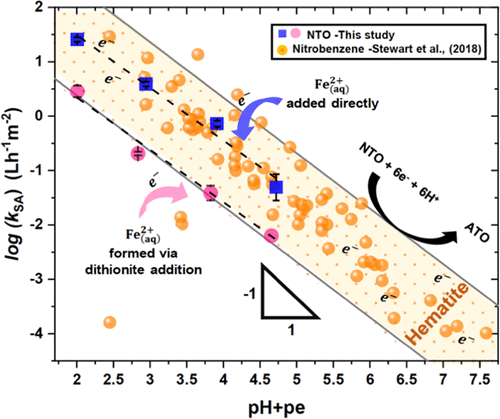
3-Nitro-1,2,4-triazol-5-one (NTO) is an insensitive munition compound (MC) that has replaced legacy MC. NTO can be highly mobile in soil and groundwater due to its high solubility and anionic nature, yet little is known about the processes that control its environmental fate. We studied NTO reduction by the hematite–Fe2+ redox couple to assess the importance of this process for the attenuation and remediation of NTO. Fe2+(aq) was either added (type I) or formed through hematite reduction by dithionite (type II). In the presence of both hematite and Fe2+(aq), NTO was quantitatively reduced to 3-amino-1,2,4-triazol-5-one following first-order kinetics. The surface area-normalized rate constant (kSA) showed a strong pH dependency between 5.5 and 7.0 and followed a linear free energy relationship (LFER) proposed in a previous study for nitrobenzene reduction by iron oxide–Fe2+ couples, i.e., log kSA = −(pe + pH) + constant. Sulfite, a major dithionite oxidation product, lowered kSA in type II system by ∼10-fold via at least two mechanisms: by complexing Fe2+ and thereby raising pe, and by making hematite more negatively charged and hence impeding NTO adsorption. This study demonstrates the importance of iron oxide–Fe2+ in controlling NTO transformation, presents an LFER for predicting NTO reduction rate, and illustrates how solutes can shift the LFER by interacting with either iron species.
中文翻译:

赤铁矿水Fe(II)氧化还原偶合剂还原3-硝基-1,2,4-三唑-5-酮(NTO)。
3-硝基1,2,4-三唑-5-酮(NTO)是一种不敏感的弹药化合物(MC),已取代了传统的MC。由于NTO的高溶解度和阴离子性质,它可以在土壤和地下水中高度移动,但对其控制环境命运的过程知之甚少。我们研究了赤铁矿-Fe 2+氧化还原对对NTO的还原作用,以评估该过程对NTO衰减和修复的重要性。添加Fe 2+ (水溶液)(I型)或通过连二亚硫酸盐还原赤铁矿形成(II型)。在赤铁矿和Fe 2+ (水溶液)的存在下,NTO均按照一级动力学定量还原为3-氨基-1,2,4-三唑-5。表面积归一化速率常数(k SA)在5.5至7.0之间显示出很强的pH依赖性,并且遵循了先前研究中提出的线性自由能关系(LFER),该氧化铁-Fe 2+对偶合还原硝基苯,即log k SA =-(pe + pH)+不变。亚硫酸氢盐是一种重要的连二亚硫酸盐氧化产物,它通过至少两种机理将II型体系中的k SA降低约10倍:通过络合Fe 2+从而提高pe,并使赤铁矿带更多负电并因此阻碍NTO吸附。这项研究证明了氧化铁– Fe 2+的重要性 在控制NTO转化过程中,我们提出了一种预测NTO还原速率的LFER,并说明了溶质如何通过与任何一种铁离子相互作用而改变LFER。
更新日期:2020-10-06
中文翻译:

赤铁矿水Fe(II)氧化还原偶合剂还原3-硝基-1,2,4-三唑-5-酮(NTO)。
3-硝基1,2,4-三唑-5-酮(NTO)是一种不敏感的弹药化合物(MC),已取代了传统的MC。由于NTO的高溶解度和阴离子性质,它可以在土壤和地下水中高度移动,但对其控制环境命运的过程知之甚少。我们研究了赤铁矿-Fe 2+氧化还原对对NTO的还原作用,以评估该过程对NTO衰减和修复的重要性。添加Fe 2+ (水溶液)(I型)或通过连二亚硫酸盐还原赤铁矿形成(II型)。在赤铁矿和Fe 2+ (水溶液)的存在下,NTO均按照一级动力学定量还原为3-氨基-1,2,4-三唑-5。表面积归一化速率常数(k SA)在5.5至7.0之间显示出很强的pH依赖性,并且遵循了先前研究中提出的线性自由能关系(LFER),该氧化铁-Fe 2+对偶合还原硝基苯,即log k SA =-(pe + pH)+不变。亚硫酸氢盐是一种重要的连二亚硫酸盐氧化产物,它通过至少两种机理将II型体系中的k SA降低约10倍:通过络合Fe 2+从而提高pe,并使赤铁矿带更多负电并因此阻碍NTO吸附。这项研究证明了氧化铁– Fe 2+的重要性 在控制NTO转化过程中,我们提出了一种预测NTO还原速率的LFER,并说明了溶质如何通过与任何一种铁离子相互作用而改变LFER。


























 京公网安备 11010802027423号
京公网安备 11010802027423号