当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Catalytic Regioselective Benzoylation of 1,2-trans-Diols in Carbohydrates with Benzoyl Cyanide: The Axial Oxy Group Effect and the Action of Achiral and Chiral Amine Catalysts
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-09-09 , DOI: 10.1021/acscatal.0c02112 Tianlu Li 1, 2 , Tong Li 1 , Michael Linseis 3 , Fengshan Wang 1, 2 , Rainer F. Winter 3 , Richard R. Schmidt 3 , Peng Peng 1, 2
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-09-09 , DOI: 10.1021/acscatal.0c02112 Tianlu Li 1, 2 , Tong Li 1 , Michael Linseis 3 , Fengshan Wang 1, 2 , Rainer F. Winter 3 , Richard R. Schmidt 3 , Peng Peng 1, 2
Affiliation
|
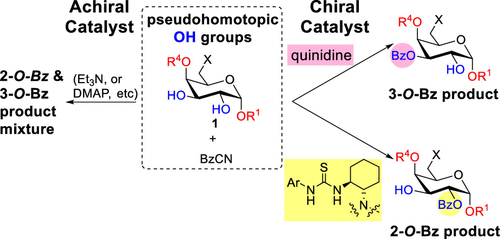
Regioselective protection of the polyfunctional carbohydrates with acyl groups under catalytic conditions is a prerequisite for efficient structural modification and chain extension via glycosidation. A general and also strong “axial oxy group effect” was now observed with benzoyl cyanide as the acylating agent and 4-dimethylaminopyridine as the catalyst, permitting the preferred O-acylation of equatorial hydroxy groups next to axial oxy groups. This effect is substantiated with 2,3-O-unprotected β-d-galacto- and α-d-glucopyranosides and 3,4-O-unprotected mannopyranosides possessing vicinal trans-diol moieties. Moreover, vicinal trans-diols with axial oxy groups next to each hydroxy group, possessing expectedly comparable reactivity, could be differentiated with chiral tertiary amine catalysts. Particularly interesting in this regard is the action of bifunctional (S,S)-N-(N,N-dialkylaminocyclohexyl)-thioureas as catalysts; they favor 2-O-benzoylation of 2,3-O-unprotected α-galactopyranosides, as is also supported by density functional theory calculations. This directing effect can be reversed with quinidine as the catalyst or with bulky substituents at the anomeric position.
中文翻译:

苯甲酰氰化物催化1,2-反式二酚在碳水化合物中的区域选择性苯甲酰化:轴向氧基团的作用以及手性和手性胺催化剂的作用
在催化条件下用酰基对多官能碳水化合物进行区域选择性保护是通过糖苷化进行有效结构修饰和链扩展的前提条件。现在观察到一般且强烈的“轴向氧基团效应”,其中苯甲酰氰化物为酰化剂,并且4-二甲基氨基吡啶为催化剂,允许优选在轴向氧基旁边的赤道羟基的O-酰化。该作用被具有邻位反式-二醇部分的2,3- O-未保护的β- d-半乳糖和α- d-吡喃葡糖苷和3,4- O-未保护的甘露吡喃糖苷证实。而且,邻近的反式具有手性叔胺催化剂可以区分具有预期可比的反应性的,轴向羟基紧挨着每个羟基的二醇。在这方面特别令人感兴趣的是双官能(S,S)-N-(N,N-二烷基氨基环己基)-硫脲作为催化剂的作用。他们赞成2,3 - O-未保护的α-吡喃半乳糖苷的2- O-苯甲酰化,这也得到密度泛函理论计算的支持。用奎尼丁作为催化剂或在异头位置上有大体积的取代基可以逆转这种导向作用。
更新日期:2020-10-02
中文翻译:

苯甲酰氰化物催化1,2-反式二酚在碳水化合物中的区域选择性苯甲酰化:轴向氧基团的作用以及手性和手性胺催化剂的作用
在催化条件下用酰基对多官能碳水化合物进行区域选择性保护是通过糖苷化进行有效结构修饰和链扩展的前提条件。现在观察到一般且强烈的“轴向氧基团效应”,其中苯甲酰氰化物为酰化剂,并且4-二甲基氨基吡啶为催化剂,允许优选在轴向氧基旁边的赤道羟基的O-酰化。该作用被具有邻位反式-二醇部分的2,3- O-未保护的β- d-半乳糖和α- d-吡喃葡糖苷和3,4- O-未保护的甘露吡喃糖苷证实。而且,邻近的反式具有手性叔胺催化剂可以区分具有预期可比的反应性的,轴向羟基紧挨着每个羟基的二醇。在这方面特别令人感兴趣的是双官能(S,S)-N-(N,N-二烷基氨基环己基)-硫脲作为催化剂的作用。他们赞成2,3 - O-未保护的α-吡喃半乳糖苷的2- O-苯甲酰化,这也得到密度泛函理论计算的支持。用奎尼丁作为催化剂或在异头位置上有大体积的取代基可以逆转这种导向作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号