Biomaterials Advances ( IF 7.9 ) Pub Date : 2020-09-09 , DOI: 10.1016/j.msec.2020.111494 Amin Amani , Mohammad Reza Alizadeh , Hashem Yaghoubi , Mahdi Nohtani
|
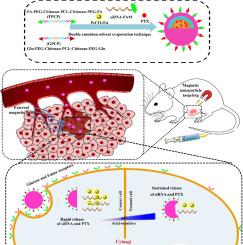
Selective delivery of drugs to damaged tissues favorable to reduce the side effects while enhancing the therapeutic efficacy. The purpose of the present study was the design and synthesis of multi-targeted nanoparticles for co-delivery of both drug and nucleic acid to cancer cells. In the present study biocompatible compounds such as chitosan, polyethylene glycol (PEG), polycaprolactone (PCL), folic acid (FA) and glucose (Glu) were used to synthesize the FA-PEG-Chitosan-PCL-Chitosan-PEG-FA (FPCP) and Glu-PEG-Chitosan-PCL-Chitosan-PEG-Glu (GPCP) copolymers. Then, paclitaxel (PTX), oleic acid-coated FeCO nanoparticles (FeCO-OA) and 6-carboxy-fluorescein phosphoramidate (FAM)-labeled siRNA (siRNA-FAM) were encapsulated into either FPCP or GPCP, or both FPCP and GPCP (GFPCP), using the solvent evaporation technique.
In vitro and in vivo biocompatibility and drug delivery efficiency of FPCP/FeCO-OA/PTX, GPCP/FeCO-OA/PTX and GFPCP/FeCO-OA/PTX nanoparticles were determined by recording the MTT assay, weight loss and tumor volume respectively. In addition, the ability of FPCP/FeCO-OA/siRNA-FAM, GPCP/FeCO-OA/siRNA-FAM, and GFPCP/FeCO-OA/siRNA-FAM gene transfer was determined using flow cytometry analysis. Moreover, the effects of applying an external magnetic field to the tumor site on the efficiency of drug delivery using FPCP/FeCO-OA/siRNA-FAM/PTX (NPsA), GPCP/FeCO-OA/siRNA-FAM/PTX (NPsB) and GFPCP/FeCO-OA/siRNA-FAM/PTX (NPsAB) were also investigated in the present study.
No significant toxicity was observed for the FPCP and GPCP. Meanwhile, PTX encapsulated FPCP, GPCP and GFPCP exhibited greater anticancer properties against MCF-7 cells. The in vivo and in vitro results showed that the nanoparticles targeted with both folic acid and glucose increased drug and RNA transfer efficiency compared to when folic acid or glucose alone used. Also, the efficiency of PTX and siRNA-FAM delivery to tumor tissues by nanoparticles increased significantly by applying an external magnetic field to the tumor area. The hydrophobic interactions between different amphipathic copolymers in appropriate is an efficient and easy technique to synthesize complex and multifunctional nanoparticles.
中文翻译:

新型多靶点纳米粒子,可将核酸和化学治疗剂靶向共递送至乳腺癌组织
药物向受损组织的选择性递送有利于减少副作用,同时增强治疗功效。本研究的目的是设计和合成可将药物和核酸共同递送至癌细胞的多靶点纳米粒子。在本研究中,使用生物相容性化合物如壳聚糖,聚乙二醇(PEG),聚己内酯(PCL),叶酸(FA)和葡萄糖(Glu)来合成FA-PEG-壳聚糖-PCL-壳聚糖-PEG-FA( FPCP)和Glu-PEG-壳聚糖-PCL-壳聚糖-PEG-Glu(GPCP)共聚物。然后,将紫杉醇(PTX),油酸涂覆的FeCO纳米颗粒(FeCO-OA)和6-羧基荧光素氨基磷酸酯(FAM)标记的siRNA(siRNA-FAM)封装到FPCP或GPCP中,或者将FPCP和GPCP封装在一起( GFPCP),使用溶剂蒸发技术。
FPCP / FeCO-OA / PTX,GPCP / FeCO-OA / PTX和GFPCP / FeCO-OA / PTX纳米粒子的体外和体内生物相容性和药物传递效率分别通过记录MTT分析,重量减轻和肿瘤体积来确定。另外,使用流式细胞术分析确定了FPCP / FeCO-OA / siRNA-FAM,GPCP / FeCO-OA / siRNA-FAM和GFPCP / FeCO-OA / siRNA-FAM基因转移的能力。此外,使用FPCP / FeCO-OA / siRNA-FAM / PTX(NPsA),GPCP / FeCO-OA / siRNA-FAM / PTX(NPsB)向肿瘤部位施加外部磁场对药物输送效率的影响GFPCP / FeCO-OA / siRNA-FAM / PTX(NPsAB)也在本研究中进行了研究。
对于FPCP和GPCP没有观察到明显的毒性。同时,PTX封装的FPCP,GPCP和GFPCP对MCF-7细胞表现出更大的抗癌特性。体内和体外结果表明,与单独使用叶酸或葡萄糖时相比,以叶酸和葡萄糖为靶标的纳米颗粒可提高药物和RNA的转移效率。同样,通过向肿瘤区域施加外部磁场,纳米粒子将PTX和siRNA-FAM递送至肿瘤组织的效率显着提高。适当地,不同两亲共聚物之间的疏水相互作用是合成复杂和多功能纳米颗粒的有效且容易的技术。


























 京公网安备 11010802027423号
京公网安备 11010802027423号