当前位置:
X-MOL 学术
›
Hydrometallurgy
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hydrometallurgical stepwise recovery of copper and zinc from smelting slag of waste brass in ammonium chloride solution
Hydrometallurgy ( IF 4.7 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.hydromet.2020.105475 Zhimei Xia , Xiaosa Zhang , Xinglong Huang , Shenghai Yang , Yongming Chen , Longgang Ye
Hydrometallurgy ( IF 4.7 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.hydromet.2020.105475 Zhimei Xia , Xiaosa Zhang , Xinglong Huang , Shenghai Yang , Yongming Chen , Longgang Ye
|
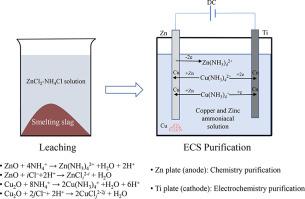
Abstract A new process for recycling zinc and copper from the smelting slag of waste brass was investigated in this study. The zinc and copper present in the smelting slag were dissolved in a ZnCl2–NH4Cl solution system. To recycle copper, the lixivium was purified by a novel method termed as electrochemistry and chemistry synergetic (ECS) purification. After deep purification of the ECS-treated lixivium by zinc powder cementation, zinc in the lixivium was extracted by electrowinning to obtain a zinc plate. The effects of the leaching temperature, reaction time, and liquid–solid ratio on the extraction percentages of zinc and copper were examined. The results revealed that the extraction percentages of zinc and copper were 88.37% and 90.85%, respectively, at a leaching temperature of 95 °C, reaction time of 90 min, and liquid–solid ratio of 8:1 mL/g. Further, effects of the current density, temperature, and reaction time on the recovery ratio of copper by ECS method were examined. The results revealed that 98.51% of copper could be extracted from the lixivium and that the purity of the extracted copper was 96.7% at a temperature of 70 °C and cathode current density of 75 A/m2 after a reaction for 4 h. In the deep purification process, 0.3 g/L zinc powder was used to completely remove impurities from the ECS-treated lixivium. In the zinc electrowinning process, a current efficiency of 95.79% was achieved at 70 °C and 400 A/m2; the purity of the obtained zinc product was greater than 99.95%, and the direct energy consumption was 2966.59 kW·h/t-Zn.
中文翻译:

氯化铵溶液中废黄铜冶炼渣湿法冶金逐步回收铜和锌
摘要 研究了一种从废黄铜冶炼渣中回收锌和铜的新工艺。熔炼渣中的锌和铜溶解在 ZnCl2-NH4Cl 溶液系统中。为了回收铜,浸出液通过一种称为电化学和化学协同 (ECS) 纯化的新方法进行纯化。通过锌粉渗碳对ECS处理的浸出液进行深度净化后,通过电积提取浸出液中的锌以获得锌板。考察了浸出温度、反应时间和液固比对锌和铜提取率的影响。结果表明,浸出温度为95℃,反应时间为90min,液固比为8时,锌和铜的提取率分别为88.37%和90.85%:1毫升/克。此外,研究了电流密度、温度和反应时间对 ECS 法铜回收率的影响。结果表明,在70°C的温度和75 A/m2的阴极电流密度下,反应4小时后,可以从浸出液中提取98.51%的铜,提取的铜纯度为96.7%。在深度纯化过程中,使用 0.3 g/L 的锌粉完全去除 ECS 处理过的浸出液中的杂质。在锌电积过程中,在70℃和400A/m2下电流效率达到95.79%;所得锌产品纯度大于99.95%,直接能耗为2966.59 kW·h/t-Zn。结果表明,在70°C的温度和75 A/m2的阴极电流密度下,反应4小时后,可以从浸出液中提取98.51%的铜,提取铜的纯度为96.7%。在深度纯化过程中,使用 0.3 g/L 的锌粉完全去除 ECS 处理过的浸出液中的杂质。在锌电积过程中,在70℃和400A/m2下电流效率达到95.79%;所得锌产品纯度大于99.95%,直接能耗为2966.59 kW·h/t-Zn。结果表明,在70°C的温度和75 A/m2的阴极电流密度下,反应4小时后,可以从浸出液中提取98.51%的铜,提取的铜纯度为96.7%。在深度纯化过程中,使用 0.3 g/L 的锌粉完全去除 ECS 处理过的浸出液中的杂质。在锌电积过程中,在70℃和400A/m2下电流效率达到95.79%;所得锌产品纯度大于99.95%,直接能耗为2966.59 kW·h/t-Zn。使用 3 g/L 锌粉从 ECS 处理的浸出液中完全去除杂质。在锌电积过程中,在70℃和400A/m2下电流效率达到95.79%;所得锌产品纯度大于99.95%,直接能耗为2966.59 kW·h/t-Zn。使用 3 g/L 锌粉从 ECS 处理的浸出液中完全去除杂质。在锌电积过程中,在70℃和400A/m2下电流效率达到95.79%;所得锌产品纯度大于99.95%,直接能耗为2966.59 kW·h/t-Zn。
更新日期:2020-11-01
中文翻译:

氯化铵溶液中废黄铜冶炼渣湿法冶金逐步回收铜和锌
摘要 研究了一种从废黄铜冶炼渣中回收锌和铜的新工艺。熔炼渣中的锌和铜溶解在 ZnCl2-NH4Cl 溶液系统中。为了回收铜,浸出液通过一种称为电化学和化学协同 (ECS) 纯化的新方法进行纯化。通过锌粉渗碳对ECS处理的浸出液进行深度净化后,通过电积提取浸出液中的锌以获得锌板。考察了浸出温度、反应时间和液固比对锌和铜提取率的影响。结果表明,浸出温度为95℃,反应时间为90min,液固比为8时,锌和铜的提取率分别为88.37%和90.85%:1毫升/克。此外,研究了电流密度、温度和反应时间对 ECS 法铜回收率的影响。结果表明,在70°C的温度和75 A/m2的阴极电流密度下,反应4小时后,可以从浸出液中提取98.51%的铜,提取的铜纯度为96.7%。在深度纯化过程中,使用 0.3 g/L 的锌粉完全去除 ECS 处理过的浸出液中的杂质。在锌电积过程中,在70℃和400A/m2下电流效率达到95.79%;所得锌产品纯度大于99.95%,直接能耗为2966.59 kW·h/t-Zn。结果表明,在70°C的温度和75 A/m2的阴极电流密度下,反应4小时后,可以从浸出液中提取98.51%的铜,提取铜的纯度为96.7%。在深度纯化过程中,使用 0.3 g/L 的锌粉完全去除 ECS 处理过的浸出液中的杂质。在锌电积过程中,在70℃和400A/m2下电流效率达到95.79%;所得锌产品纯度大于99.95%,直接能耗为2966.59 kW·h/t-Zn。结果表明,在70°C的温度和75 A/m2的阴极电流密度下,反应4小时后,可以从浸出液中提取98.51%的铜,提取的铜纯度为96.7%。在深度纯化过程中,使用 0.3 g/L 的锌粉完全去除 ECS 处理过的浸出液中的杂质。在锌电积过程中,在70℃和400A/m2下电流效率达到95.79%;所得锌产品纯度大于99.95%,直接能耗为2966.59 kW·h/t-Zn。使用 3 g/L 锌粉从 ECS 处理的浸出液中完全去除杂质。在锌电积过程中,在70℃和400A/m2下电流效率达到95.79%;所得锌产品纯度大于99.95%,直接能耗为2966.59 kW·h/t-Zn。使用 3 g/L 锌粉从 ECS 处理的浸出液中完全去除杂质。在锌电积过程中,在70℃和400A/m2下电流效率达到95.79%;所得锌产品纯度大于99.95%,直接能耗为2966.59 kW·h/t-Zn。


























 京公网安备 11010802027423号
京公网安备 11010802027423号