当前位置:
X-MOL 学术
›
Comp. Mater. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structure and dynamics of aqueous solutions containing poly-(acrylic acid) and non-ionic surfactant pentaethylene glycol n-octyl ether (C8E5): A molecular simulations study
Computational Materials Science ( IF 3.3 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.commatsci.2020.110043 Lakshmikumar Kunche , Upendra Natarajan
Computational Materials Science ( IF 3.3 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.commatsci.2020.110043 Lakshmikumar Kunche , Upendra Natarajan
|
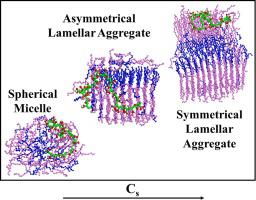
Abstract Self-assembly of non-ionic surfactant pentaethylene glycol n-octyl ether (C8E5) in aqueous solution containing anionic polyelectrolyte poly (acrylic acid) PAA was investigated via atomistic molecular dynamics (MD) simulations. The concentration of surfactant (Cs) varied in the range (Cs = 9.6 × 10−4–0.3 M) of dilute to concentrated systems while polymer concentration was in dilute solution condition (i.e. Cp = 0.01 M). Binary and ternary systems show formation of nearly spherical micellar aggregate at low Cs and lamellar aggregates at high Cs. Strong interaction between PAA and C8E5 is observed at low Cs, in agreement with experimental data. PAA chain adsorbs onto the micellar aggregate and wraps around the aggregate. For lamellar phase aggregates PAA chain shows polar interactions with hydrophilic moieties of surfactant, originating from interactions between COOH groups of PAA and CO groups of C8E5. The concentration at which free-micelles form in ternary (polymer, surfactant and water) solution is greater than CMC of the binary surfactant-water system, in qualitative agreement with experimental data. At low Cs the interaction between surfactants and polymer chain is weak and does not perturb chain conformations. The radius-of-gyration Rg of PAA increases with Cs. Interaction of surfactant molecules with water is more cooperative at higher values of surfactant concentration, as evident from the values of solvation enthalpy. Significant hydrogen bonds were observed to occur between polar oxygens and hydroxyl oxygen (O2HA) of PAA. The number of h-bonds between polar oxygens and O2HA of PAA increase with Cs. Hydrogen bonds of PAA with surfactant molecules increases with Cs as the polymer expands at high Cs. Formation of surfactant aggregate is enthalpically favored across the entire range of concentration in binary and ternary solution. Binding of PAA to surfactant aggregate is favorable at higher surfactant concentration. The enthalpy of formation of ternary solution exhibits a transition from unfavorable to favorable which interestingly is driven by the structural transition from spherical micellar to the lamellar phase. This signature for this transition is also seen by conformation change of PAA upon binding to surfactant aggregate.
中文翻译:

含有聚(丙烯酸)和非离子表面活性剂五乙二醇正辛基醚 (C8E5) 的水溶液的结构和动力学:分子模拟研究
摘要 通过原子分子动力学 (MD) 模拟研究了非离子表面活性剂五乙二醇正辛基醚 (C8E5) 在含有阴离子聚电解质聚(丙烯酸)PAA 的水溶液中的自组装。表面活性剂 (Cs) 的浓度在稀释到浓缩系统的范围 (Cs = 9.6 × 10−4–0.3 M) 内变化,而聚合物浓度处于稀溶液条件 (即 Cp = 0.01 M)。二元和三元系统显示在低 Cs 下形成近乎球形的胶束聚集体,在高 Cs 下形成层状聚集体。在低 Cs 下观察到 PAA 和 C8E5 之间的强相互作用,与实验数据一致。PAA 链吸附在胶束聚集体上并包裹在聚集体周围。对于层状相聚集体,PAA 链显示出与表面活性剂亲水部分的极性相互作用,源于 PAA 的 COOH 基团和 C8E5 的 CO 基团之间的相互作用。在三元(聚合物、表面活性剂和水)溶液中形成游离胶束的浓度大于二元表面活性剂-水体系的 CMC,与实验数据定性一致。在低 Cs 下,表面活性剂和聚合物链之间的相互作用很弱,不会扰乱链的构象。PAA 的回转半径 Rg 随 Cs 增加。表面活性剂分子与水的相互作用在较高的表面活性剂浓度值下更加协同,这从溶剂化焓值中可以明显看出。观察到在极性氧和 PAA 的羟基氧 (O2HA) 之间发生显着的氢键。PAA 的极性氧和 O2HA 之间的 h 键数量随着 Cs 的增加而增加。随着聚合物在高 Cs 下膨胀,PAA 与表面活性剂分子的氢键随 Cs 增加。在二元和三元溶液的整个浓度范围内,表面活性剂聚集体的形成在焓上是有利的。在较高的表面活性剂浓度下,PAA 与表面活性剂聚集体的结合是有利的。三元溶液的形成焓表现出从不利到有利的转变,有趣的是,这是由球形胶束到层状相的结构转变所驱动的。通过与表面活性剂聚集体结合后 PAA 的构象变化也可以看出这种转变的特征。在较高的表面活性剂浓度下,PAA 与表面活性剂聚集体的结合是有利的。三元溶液的形成焓表现出从不利到有利的转变,有趣的是,这是由球形胶束到层状相的结构转变所驱动的。通过与表面活性剂聚集体结合后 PAA 的构象变化也可以看出这种转变的特征。在较高的表面活性剂浓度下,PAA 与表面活性剂聚集体的结合是有利的。三元溶液的形成焓表现出从不利到有利的转变,有趣的是,这是由球形胶束到层状相的结构转变所驱动的。通过与表面活性剂聚集体结合后 PAA 的构象变化也可以看出这种转变的特征。
更新日期:2021-01-01
中文翻译:

含有聚(丙烯酸)和非离子表面活性剂五乙二醇正辛基醚 (C8E5) 的水溶液的结构和动力学:分子模拟研究
摘要 通过原子分子动力学 (MD) 模拟研究了非离子表面活性剂五乙二醇正辛基醚 (C8E5) 在含有阴离子聚电解质聚(丙烯酸)PAA 的水溶液中的自组装。表面活性剂 (Cs) 的浓度在稀释到浓缩系统的范围 (Cs = 9.6 × 10−4–0.3 M) 内变化,而聚合物浓度处于稀溶液条件 (即 Cp = 0.01 M)。二元和三元系统显示在低 Cs 下形成近乎球形的胶束聚集体,在高 Cs 下形成层状聚集体。在低 Cs 下观察到 PAA 和 C8E5 之间的强相互作用,与实验数据一致。PAA 链吸附在胶束聚集体上并包裹在聚集体周围。对于层状相聚集体,PAA 链显示出与表面活性剂亲水部分的极性相互作用,源于 PAA 的 COOH 基团和 C8E5 的 CO 基团之间的相互作用。在三元(聚合物、表面活性剂和水)溶液中形成游离胶束的浓度大于二元表面活性剂-水体系的 CMC,与实验数据定性一致。在低 Cs 下,表面活性剂和聚合物链之间的相互作用很弱,不会扰乱链的构象。PAA 的回转半径 Rg 随 Cs 增加。表面活性剂分子与水的相互作用在较高的表面活性剂浓度值下更加协同,这从溶剂化焓值中可以明显看出。观察到在极性氧和 PAA 的羟基氧 (O2HA) 之间发生显着的氢键。PAA 的极性氧和 O2HA 之间的 h 键数量随着 Cs 的增加而增加。随着聚合物在高 Cs 下膨胀,PAA 与表面活性剂分子的氢键随 Cs 增加。在二元和三元溶液的整个浓度范围内,表面活性剂聚集体的形成在焓上是有利的。在较高的表面活性剂浓度下,PAA 与表面活性剂聚集体的结合是有利的。三元溶液的形成焓表现出从不利到有利的转变,有趣的是,这是由球形胶束到层状相的结构转变所驱动的。通过与表面活性剂聚集体结合后 PAA 的构象变化也可以看出这种转变的特征。在较高的表面活性剂浓度下,PAA 与表面活性剂聚集体的结合是有利的。三元溶液的形成焓表现出从不利到有利的转变,有趣的是,这是由球形胶束到层状相的结构转变所驱动的。通过与表面活性剂聚集体结合后 PAA 的构象变化也可以看出这种转变的特征。在较高的表面活性剂浓度下,PAA 与表面活性剂聚集体的结合是有利的。三元溶液的形成焓表现出从不利到有利的转变,有趣的是,这是由球形胶束到层状相的结构转变所驱动的。通过与表面活性剂聚集体结合后 PAA 的构象变化也可以看出这种转变的特征。



























 京公网安备 11010802027423号
京公网安备 11010802027423号