Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics ( IF 3.2 ) Pub Date : 2020-09-09 , DOI: 10.1016/j.bbapap.2020.140538 Juliana Jaramillo-Ramírez 1 , Nancy Marcial-Bazaldua 1 , Nuria Sánchez-Puig 2
|
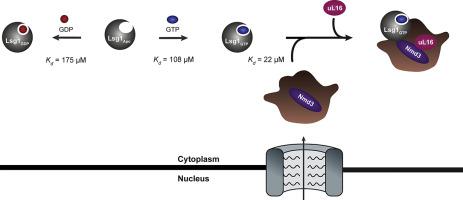
Ribosome biogenesis in eukaryotes requires the participation of several transactivation factors that are involved in the modification, assembly, transport and quality control of the ribosomal subunits. One of these factors is the Large subunit GTPase 1 (Lsg1), a protein that acts as the release factor for the export adaptor named Nonsense-mediated mRNA decay 3 protein (Nmd3) and facilitates the incorporation of the last structural protein uL16 into the 60S subunit. Here, we characterised the recombinant yeast Lsg1 and studied its catalysis and binding properties for guanine nucleotides. We described the interaction of Lsg1 with guanine nucleotides alone and in the presence of the complex Nmd3•60S using fluorescence spectroscopy. Lsg1 has a greater affinity for GTP than for GDP suggesting that in the cell cytoplasm it exists mainly bound to the former. In the presence of 60S subunits loaded with Nmd3, the affinity of Lsg1 for both nucleotides increases but to a larger extent towards GTP. From this observation together with the excess of GTP present in the cytoplasm of exponentially growing cells over that of GDP, we can infer that the pre-ribosomal particle composed by Nmd3•60S acts as a GTP Stabilising Factor for Lsg1. Additionally, Lsg1 undergoes different conformational changes depending on its binding partner or the guanine nucleotides it interacts with. Steady-state kinetic analysis of free Lsg1 indicated slow GTP hydrolysis with values of kcat 1 min−1 and Km of 34 μM.
中文翻译:

鸟嘌呤核苷酸与核糖体GTPase Lsg1相互作用的表征。
真核生物中的核糖体生物发生需要参与核糖体亚基的修饰,组装,运输和质量控制的几种反式激活因子的参与。这些因素之一是大亚基GTPase 1(Lsg1),该蛋白质充当输出适配器的输出因子,称为无意义介导的mRNA衰变3蛋白(Nmd3),并有助于将最后的结构蛋白uL16掺入60S中。亚基。在这里,我们表征了重组酵母Lsg1,并研究了其对鸟嘌呤核苷酸的催化和结合特性。我们描述了Lsg1与鸟嘌呤核苷酸的相互作用以及在复杂的Nmd3•60S存在下的荧光光谱分析。Lsg1对GTP的亲和力大于对GDP的亲和力,这表明在细胞质中它主要与前者结合。在存在Nmd3的60S亚基的情况下,Lsg1对两个核苷酸的亲和力都会增加,但对GTP的影响更大。从这个观察结果来看,加上指数生长细胞的细胞质中的GTP超过GDP的过量,我们可以推断出Nmd3•60S组成的核糖体前颗粒充当Lsg1的GTP稳定因子。此外,Lsg1根据其结合伴侣或与其相互作用的鸟嘌呤核苷酸而经历不同的构象变化。游离Lsg1的稳态动力学分析表明GTP水解缓慢,其值为 Lsg1对两个核苷酸的亲和力增加,但对GTP的程度更大。从这个观察结果来看,加上指数生长细胞的细胞质中的GTP超过GDP的过量,我们可以推断出Nmd3•60S组成的核糖体前颗粒充当Lsg1的GTP稳定因子。此外,Lsg1根据其结合伴侣或与其相互作用的鸟嘌呤核苷酸而经历不同的构象变化。游离Lsg1的稳态动力学分析表明GTP水解缓慢,其值为 Lsg1对两个核苷酸的亲和力增加,但对GTP的程度更大。从这个观察结果来看,加上指数生长细胞的细胞质中的GTP超过GDP的过量,我们可以推断出Nmd3•60S组成的核糖体前颗粒充当Lsg1的GTP稳定因子。此外,Lsg1根据其结合伴侣或与其相互作用的鸟嘌呤核苷酸而经历不同的构象变化。游离Lsg1的稳态动力学分析表明GTP水解缓慢,其值为 Lsg1经历不同的构象变化,具体取决于其结合伴侣或与之相互作用的鸟嘌呤核苷酸。游离Lsg1的稳态动力学分析表明GTP水解缓慢,其值为 Lsg1经历不同的构象变化,具体取决于其结合伴侣或与之相互作用的鸟嘌呤核苷酸。游离Lsg1的稳态动力学分析表明GTP水解缓慢,其值为k cat 1 min -1和K m为34μM。



























 京公网安备 11010802027423号
京公网安备 11010802027423号