当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Five-membered cyclic sulfamidate imines: versatile scaffolds for organic synthesis.
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-09-08 , DOI: 10.1039/d0ob01568g Quoc Hoang Pham 1 , Christopher J T Hyland , Stephen G Pyne
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-09-08 , DOI: 10.1039/d0ob01568g Quoc Hoang Pham 1 , Christopher J T Hyland , Stephen G Pyne
Affiliation
|
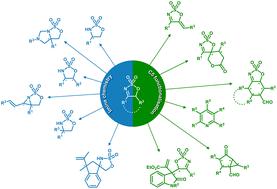
In recent years, five-membered ring cyclic sulfamidate imines (5H-1,2,3-oxathiazole 2,2-dioxides) have received increasing attention as useful precursors for the stereoselective synthesis of many valuable heterocycles. Bearing a reactive N-sulfonyl imine moiety as part of the stereodefined skeleton, this sulfamidate imine platform has been utilised as a substrate in many reactions, including nucleophilic additions and reductions, to prepare highly functionalised cyclic sulfamidates. In addition, cyclic sulfamidate imines can also readily participate as nucleophiles in many chemical transformations, owing to the acidic proton(s) adjacent to the imine moiety. This short review highlights recent developments involving cyclic sulfamidate imines, including their synthesis and reactivity, with a focus on stereoselective processes.
中文翻译:

五元环状氨基磺酸亚胺:用于有机合成的多功能支架。
近年来,五元环状氨基磺酸亚胺(5 H -1,2,3-恶噻唑2,2-二氧化物)作为立体选择性合成许多有价值的杂环的有用前体而受到越来越多的关注。轴承无功N-磺酰亚胺部分作为立体定义骨架的一部分,这种氨基磺酸盐亚胺平台已被用作许多反应中的底物,包括亲核加成和还原,以制备高度官能化的环状氨基磺酸盐。此外,由于与亚胺部分相邻的酸性质子,环状氨基磺酸亚胺也可以很容易地作为亲核试剂参与许多化学转化。这篇简短的综述重点介绍了环状氨基磺酸亚胺的最新发展,包括它们的合成和反应性,重点是立体选择性过程。
更新日期:2020-10-07
中文翻译:

五元环状氨基磺酸亚胺:用于有机合成的多功能支架。
近年来,五元环状氨基磺酸亚胺(5 H -1,2,3-恶噻唑2,2-二氧化物)作为立体选择性合成许多有价值的杂环的有用前体而受到越来越多的关注。轴承无功N-磺酰亚胺部分作为立体定义骨架的一部分,这种氨基磺酸盐亚胺平台已被用作许多反应中的底物,包括亲核加成和还原,以制备高度官能化的环状氨基磺酸盐。此外,由于与亚胺部分相邻的酸性质子,环状氨基磺酸亚胺也可以很容易地作为亲核试剂参与许多化学转化。这篇简短的综述重点介绍了环状氨基磺酸亚胺的最新发展,包括它们的合成和反应性,重点是立体选择性过程。

























 京公网安备 11010802027423号
京公网安备 11010802027423号