当前位置:
X-MOL 学术
›
Nano Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Acto-Myosin Cross-Bridge Stiffness Depends on the Nucleotide State of Myosin II.
Nano Letters ( IF 10.8 ) Pub Date : 2020-09-08 , DOI: 10.1021/acs.nanolett.0c02960 Tianbang Wang 1 , Bernhard Brenner 1 , Arnab Nayak 1 , Mamta Amrute-Nayak 1
Nano Letters ( IF 10.8 ) Pub Date : 2020-09-08 , DOI: 10.1021/acs.nanolett.0c02960 Tianbang Wang 1 , Bernhard Brenner 1 , Arnab Nayak 1 , Mamta Amrute-Nayak 1
Affiliation
|
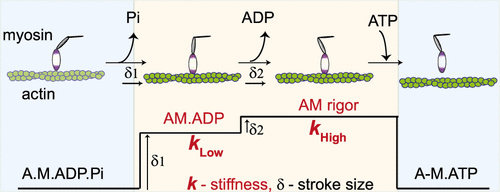
How various myosin isoforms fulfill the diverse physiological requirements of distinct muscle types remain unclear. Myosin II isoforms expressed in skeletal muscles determine the mechanical performance of the specific muscles. Here, we employed a single-molecule optical trapping method and compared the chemomechanical properties of slow and fast muscle myosin II isoforms. Stiffness of the myosin motor is key to its force-generating ability during muscle contraction. We found that acto-myosin (AM) cross-bridge stiffness depends on its nucleotide state as the myosin progresses through the ATPase cycle. The strong actin bound “AM.ADP” state exhibited >2 fold lower stiffness than “AM rigor” state. The two myosin isoforms displayed similar “rigor” stiffness. We conclude that the time-averaged stiffness of the slow myosin is lower due to prolonged duration of the AM.ADP state, which determines the force-generating potential and contraction speed of the muscle, elucidating the basis for functional diversity among myosins.
中文翻译:

Acto-Myosin跨桥刚度取决于Myosin II的核苷酸状态。
尚不清楚各种肌球蛋白同工型如何满足不同肌肉类型的多种生理要求。在骨骼肌中表达的肌球蛋白II同工型决定了特定肌肉的机械性能。在这里,我们采用了一种单分子光阱法,并比较了慢肌和快肌肌球蛋白II亚型的化学机械性质。肌球蛋白运动的刚度是其在肌肉收缩期间产生力的能力的关键。我们发现肌球蛋白(AM)跨桥刚度取决于肌球蛋白通过ATPase循环的过程中其核苷酸状态。与“ AM严谨”状态相比,强肌动蛋白结合的“ AM.ADP”状态显示出的硬度低2倍以上。两种肌球蛋白同工型显示出相似的“严格”刚度。
更新日期:2020-10-15
中文翻译:

Acto-Myosin跨桥刚度取决于Myosin II的核苷酸状态。
尚不清楚各种肌球蛋白同工型如何满足不同肌肉类型的多种生理要求。在骨骼肌中表达的肌球蛋白II同工型决定了特定肌肉的机械性能。在这里,我们采用了一种单分子光阱法,并比较了慢肌和快肌肌球蛋白II亚型的化学机械性质。肌球蛋白运动的刚度是其在肌肉收缩期间产生力的能力的关键。我们发现肌球蛋白(AM)跨桥刚度取决于肌球蛋白通过ATPase循环的过程中其核苷酸状态。与“ AM严谨”状态相比,强肌动蛋白结合的“ AM.ADP”状态显示出的硬度低2倍以上。两种肌球蛋白同工型显示出相似的“严格”刚度。



























 京公网安备 11010802027423号
京公网安备 11010802027423号