当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Metal-Free, Redox-Neutral, Site-Selective Access to Heteroarylamine via Direct Radical-Radical Cross-Coupling Powered by Visible Light Photocatalysis
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-09-08 , DOI: 10.1021/jacs.0c07600 Chao Zhou 1, 2 , Tao Lei 1, 2 , Xiang-Zhu Wei 1, 2 , Chen Ye 1, 2 , Zan Liu 1, 2 , Bin Chen 1, 2 , Chen-Ho Tung 1, 2 , Li-Zhu Wu 1, 2
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-09-08 , DOI: 10.1021/jacs.0c07600 Chao Zhou 1, 2 , Tao Lei 1, 2 , Xiang-Zhu Wei 1, 2 , Chen Ye 1, 2 , Zan Liu 1, 2 , Bin Chen 1, 2 , Chen-Ho Tung 1, 2 , Li-Zhu Wu 1, 2
Affiliation
|
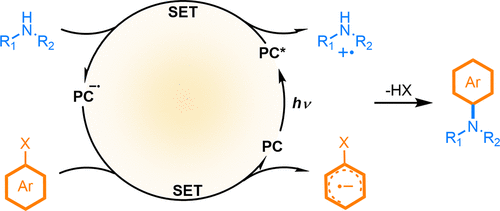
Transition-metal catalyzed C-N bond-forming reactions have emerged as fundamental and powerful tools to construct arylamines, a common structure found in drug agents, natural products and fine chemicals. Reported herein is an alternative access to heteroarylamine via radical-radical cross-coupling pathway, powered by visible light catalysis without any aid of external oxidant and reductant. Only by visible light irradiation of a photocatalyst such as a metal-free photocatalyst, a cascade single electron transfer event of amines and heteroarylnitriles, manifested by steady-state and transient spectroscopic studies, results in amine radical cation and aryl radical anion in situ for the C-N bond formation. The metal-free and redox economic nature, high efficiency and site-selectivity of C-N cross-coupling of a range of available amines, hydroxylamines and hydrazines with heteroarylnitriles make this protocol promising in both academic and industrial settings.
中文翻译:

无金属、氧化还原中性、通过可见光光催化驱动的直接自由基交叉偶联获得杂芳胺
过渡金属催化的 CN 键形成反应已成为构建芳胺的基本和强大工具,芳胺是药物、天然产物和精细化学品中的常见结构。本文报道了一种通过自由基-自由基交叉偶联途径获得杂芳胺的替代途径,由可见光催化提供动力,无需任何外部氧化剂和还原剂的帮助。只有通过光催化剂(如无金属光催化剂)的可见光照射,胺和杂芳基腈的级联单电子转移事件,通过稳态和瞬态光谱研究证明,在原位产生胺自由基阳离子和芳基阴离子CN 键的形成。一系列可用胺的 CN 交叉偶联的无金属和氧化还原经济性质、高效率和位点选择性,
更新日期:2020-09-08
中文翻译:

无金属、氧化还原中性、通过可见光光催化驱动的直接自由基交叉偶联获得杂芳胺
过渡金属催化的 CN 键形成反应已成为构建芳胺的基本和强大工具,芳胺是药物、天然产物和精细化学品中的常见结构。本文报道了一种通过自由基-自由基交叉偶联途径获得杂芳胺的替代途径,由可见光催化提供动力,无需任何外部氧化剂和还原剂的帮助。只有通过光催化剂(如无金属光催化剂)的可见光照射,胺和杂芳基腈的级联单电子转移事件,通过稳态和瞬态光谱研究证明,在原位产生胺自由基阳离子和芳基阴离子CN 键的形成。一系列可用胺的 CN 交叉偶联的无金属和氧化还原经济性质、高效率和位点选择性,


























 京公网安备 11010802027423号
京公网安备 11010802027423号