当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Methane Borylation Catalyzed by Ru, Rh, and Ir Complexes in Comparison with Cyclohexane Borylation: Theoretical Understanding and Prediction
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-09-08 , DOI: 10.1021/jacs.0c07239 Rong-Lin Zhong 1 , Shigeyoshi Sakaki 2
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-09-08 , DOI: 10.1021/jacs.0c07239 Rong-Lin Zhong 1 , Shigeyoshi Sakaki 2
Affiliation
|
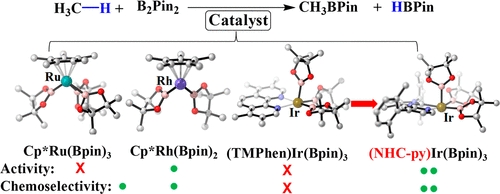
Methane borylation catalyzed by Cp*M(Bpin)n (M = Ru or Rh; HBpin = pinacolborane; n = 2 or 3) and (TMPhen)Ir(Bpin)3 (TMPhen = 3,4,7,8-tetramethyl-1,10-phenanthroline) was investigated by DFT in comparison with cyclohexane borylation. Because Ru-catalyzed borylation has not been theoretically investigated yet, its reaction mechanism was firstly elucidated; Cp*Ru(Bpin)3 1-Ru is active species and Cp*Ru(Bpin)3(H)(CH3) 4-Ru is key intermediate. In 4-Ru, the Ru is understood to have ambiguous oxidation state between +IV and +VI because they have H··Bpin bonding interaction with bond order of about 0.5. Methane borylation occurs through oxidative addition of methane C-H bond followed by reductive elimination of borylmethane in all these catalysts. Catalytic activity for methane borylation increases following the order Cp*Ru(Bpin)3 < (TMPhen)Ir(Bpin)3 < Cp*Rh(Bpin)2. Cyclohexane borylation occurs in the same mechanism except for the presence of isomerization of key intermediate. Chemoselectivity of methane over cyclohexane increases following the order Ir < Ru < Rh. In all these catalysts, the rate-determining step (RDS) of cyclohexane borylation needs larger Gº‡ than RDS of methane borylation because more bulky cyclohexyl group induces larger steric repulsion with ligand than methyl. One reason of the worst chemoselectivity of the Ir catalyst is its less congested transition state of reductive elimination of borylcyclohexane. Herein, the use of strongly electron-donating ligand consisting of pyridine and N-heterocyclic carbene with bulky substituents is computationally proposed as good ligand for the Ir catalyst; actually, the Ir complex of this ligand is calculated to be more active and more chemoselective than Cp*Rh(Bpin)2 for methane borylation.
中文翻译:

Ru、Rh 和 Ir 配合物催化的甲烷硼化与环己烷硼化的比较:理论理解和预测
由 Cp*M(Bpin)n (M = Ru 或 Rh;HBpin = 频哪醇硼烷;n = 2 或 3) 和 (TMPhen)Ir(Bpin)3 (TMPhen = 3,4,7,8-四甲基- 1,10-菲咯啉)通过 DFT 与环己烷硼酸化进行比较。由于尚未对Ru催化的硼化进行理论研究,首先阐明了其反应机理;Cp*Ru(Bpin)3 1-Ru 是活性物质,Cp*Ru(Bpin)3(H)(CH3) 4-Ru 是关键中间体。在 4-Ru 中,Ru 被理解为在 +IV 和 +VI 之间具有模糊的氧化态,因为它们具有 H··Bpin 键合相互作用,键级约为 0.5。甲烷硼酸化是通过氧化加成甲烷 CH 键,然后还原消除所有这些催化剂中的硼甲烷而发生的。甲烷硼化的催化活性按照 Cp*Ru(Bpin)3 < (TMPhen)Ir(Bpin)3 < Cp*Rh(Bpin)2。除了关键中间体的异构化之外,环己烷硼酸化以相同的机制发生。甲烷相对于环己烷的化学选择性按照 Ir < Ru < Rh 的顺序增加。在所有这些催化剂中,环己烷硼化的限速步骤 (RDS) 需要比甲烷硼化的 RDS 更大的 Gº‡,因为更大的环己基比甲基诱导更大的配体空间排斥。Ir 催化剂最差化学选择性的一个原因是其还原消除硼基环己烷的不太拥挤的过渡态。在此,计算建议使用由吡啶和 N-杂环卡宾组成的强给电子配体作为 Ir 催化剂的良好配体;实际上,
更新日期:2020-09-08
中文翻译:

Ru、Rh 和 Ir 配合物催化的甲烷硼化与环己烷硼化的比较:理论理解和预测
由 Cp*M(Bpin)n (M = Ru 或 Rh;HBpin = 频哪醇硼烷;n = 2 或 3) 和 (TMPhen)Ir(Bpin)3 (TMPhen = 3,4,7,8-四甲基- 1,10-菲咯啉)通过 DFT 与环己烷硼酸化进行比较。由于尚未对Ru催化的硼化进行理论研究,首先阐明了其反应机理;Cp*Ru(Bpin)3 1-Ru 是活性物质,Cp*Ru(Bpin)3(H)(CH3) 4-Ru 是关键中间体。在 4-Ru 中,Ru 被理解为在 +IV 和 +VI 之间具有模糊的氧化态,因为它们具有 H··Bpin 键合相互作用,键级约为 0.5。甲烷硼酸化是通过氧化加成甲烷 CH 键,然后还原消除所有这些催化剂中的硼甲烷而发生的。甲烷硼化的催化活性按照 Cp*Ru(Bpin)3 < (TMPhen)Ir(Bpin)3 < Cp*Rh(Bpin)2。除了关键中间体的异构化之外,环己烷硼酸化以相同的机制发生。甲烷相对于环己烷的化学选择性按照 Ir < Ru < Rh 的顺序增加。在所有这些催化剂中,环己烷硼化的限速步骤 (RDS) 需要比甲烷硼化的 RDS 更大的 Gº‡,因为更大的环己基比甲基诱导更大的配体空间排斥。Ir 催化剂最差化学选择性的一个原因是其还原消除硼基环己烷的不太拥挤的过渡态。在此,计算建议使用由吡啶和 N-杂环卡宾组成的强给电子配体作为 Ir 催化剂的良好配体;实际上,


























 京公网安备 11010802027423号
京公网安备 11010802027423号