当前位置:
X-MOL 学术
›
ACS Appl. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Non-Nucleophilic Electrolyte Based on Ionic Liquid and Magnesium Bis(diisopropyl)amide for Rechargeable Magnesium-Ion Batteries
ACS Applied Energy Materials ( IF 6.4 ) Pub Date : 2020-09-07 , DOI: 10.1021/acsaem.0c01026 Lethesh Kallidanthiyil Chellappan 1 , Jannicke Kvello 2 , Julian Richard Tolchard 2 , Paul Inge Dahl 2 , Sidsel Meli Hanetho 2 , Romain Berthelot 3, 4 , Anne Fiksdahl 1 , Kaushik Jayasayee 2
ACS Applied Energy Materials ( IF 6.4 ) Pub Date : 2020-09-07 , DOI: 10.1021/acsaem.0c01026 Lethesh Kallidanthiyil Chellappan 1 , Jannicke Kvello 2 , Julian Richard Tolchard 2 , Paul Inge Dahl 2 , Sidsel Meli Hanetho 2 , Romain Berthelot 3, 4 , Anne Fiksdahl 1 , Kaushik Jayasayee 2
Affiliation
|
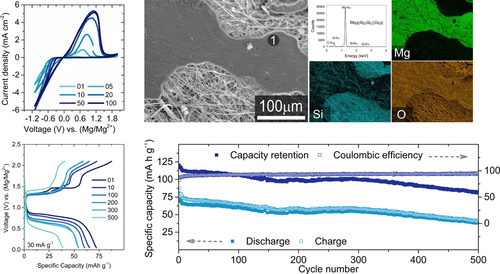
A non-nucleophilic electrolyte for rechargeable Mg-ion batteries is developed by the reaction of magnesium bis(diisopropyl)amide and 1-ethyl-3-methylimidazolium tetrachloroaluminate ionic liquid in tetrahydrofuran solvent. The electrolyte shows excellent reversibility and Coulombic efficiency for the Mg deposition/stripping process at room temperature on several working electrodes such as Mo, graphite, and stainless steel. Additionally, the electrolyte shows high anodic stability with Mo as the cathode current collector, with no corrosion detected even after 48 h at 4.5 V versus (Mg/Mg2+). An exceptional cyclability in a full cell configuration using a Chevrel phase Mo6S8 cathode is achieved with a capacity retention of more than 80% for over 300 cycles at a 15 mA g–1 specific current, making this electrolyte an excellent candidate for rechargeable Mg-ion batteries.
中文翻译:

基于离子液体和双(二异丙基)镁镁的可充电镁离子电池的非亲核电解质
通过使双(二异丙基)酰胺镁与1-乙基-3-甲基咪唑鎓四氯铝酸盐离子液体在四氢呋喃溶剂中反应,开发了用于可充电Mg离子电池的非亲核电解质。该电解质在室温下在几个工作电极(例如Mo,石墨和不锈钢)上的Mg沉积/剥离过程中显示出优异的可逆性和库仑效率。另外,电解质显示出高的阳极稳定性,以Mo作为阴极集流体,即使在4.5 V相对于(Mg / Mg 2+)48小时后也未检测到腐蚀。使用Chevrel相Mo 6 S 8在全电池配置中具有出色的循环能力在15 mA g –1的比电流下,可以在300个循环中实现80%以上的容量保持率,使这种电解质成为可充电Mg离子电池的极佳候选者。
更新日期:2020-10-26
中文翻译:

基于离子液体和双(二异丙基)镁镁的可充电镁离子电池的非亲核电解质
通过使双(二异丙基)酰胺镁与1-乙基-3-甲基咪唑鎓四氯铝酸盐离子液体在四氢呋喃溶剂中反应,开发了用于可充电Mg离子电池的非亲核电解质。该电解质在室温下在几个工作电极(例如Mo,石墨和不锈钢)上的Mg沉积/剥离过程中显示出优异的可逆性和库仑效率。另外,电解质显示出高的阳极稳定性,以Mo作为阴极集流体,即使在4.5 V相对于(Mg / Mg 2+)48小时后也未检测到腐蚀。使用Chevrel相Mo 6 S 8在全电池配置中具有出色的循环能力在15 mA g –1的比电流下,可以在300个循环中实现80%以上的容量保持率,使这种电解质成为可充电Mg离子电池的极佳候选者。


























 京公网安备 11010802027423号
京公网安备 11010802027423号